The High-cycle Fatigue Life of Cortical Bone Allografts Is Radiation Sterilization Dose-dependent: An In Vitro Study
- PMID: 35175232
- PMCID: PMC9263473
- DOI: 10.1097/CORR.0000000000002146
The High-cycle Fatigue Life of Cortical Bone Allografts Is Radiation Sterilization Dose-dependent: An In Vitro Study
Abstract
Background: Structural cortical bone allografts are a reasonable treatment option for patients with large cortical bone defects caused by trauma, tumors, or complications of arthroplasty. Although structural cortical bone allografts provide the benefit of an osteoconductive material, they are susceptible to fatigue failure (fracture) and carry a risk of disease transmission. Radiation-sterilization at the recommended dose of 25 kGy decreases the risk of disease transmission. However, previous studies demonstrated that radiation sterilization at this dose can negatively impact the high cycle-fatigue life of cortical bone. Although the effects of higher doses of radiation on cortical bone allografts are well described, the effects of lower doses of radiation on a high-cycle fatigue life of cortical bone are poorly understood.
Questions/purposes: (1) Does the cycle-fatigue life of human cortical allograft bone vary with gamma radiation dose levels of 0 (control), 10 kGy, 17.5 kGy, and 25 kGy? (2) What differences in Raman spectral biomarkers are observed following varying doses of gamma radiation exposure?
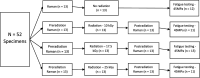
Methods: The high-cycle fatigue behavior of human cortical bone specimens was examined at different radiation sterilization doses under physiologic stress levels (35 MPa) and in a 37° C phosphate-buffered saline bath using a custom-designed rotating-bending fatigue device. Six human femora from three donors were obtained for this study (two male, 63 and 61 years old, respectively, and one female, 48 years old). Test specimens were allocated among four treatment groups (0 kGy [control], 10 kGy, 17.5 kGy, and 25 kGy) based on donor and anatomic location of harvest site (both length and cross-sectional quadrant of femoral diaphysis) to ensure equal variation (n = 13 per group). Specimens underwent high-cycle fatigue testing to failure. The number of cycles to failure was recorded. Raman spectroscopy (a noninvasive vibrational spectroscopy used to qualitatively assess bone quality) was used to detect whether any changes in Raman spectral biomarkers occurred after varying doses of gamma radiation exposure.
Results: There was a decrease in the log-transformed mean high-cycle fatigue life in specimens irradiated at 25 kGy (5.39 ± 0.32) compared with all other groups (0 kGy: 6.20 ± 0.50; 10k Gy: 6.35 ± 0.79; 17.5 kGy: 6.01 ± 0.53; p = 0.001). Specimens irradiated at 25 kGy were also more likely to exhibit a more brittle fracture surface pattern than specimens with more ductile fracture surface patterns irradiated at 0 kGy, 10 kGy, and 17.5 kGy (p = 0.04). The Raman biomarker for the ratio of the relative amount of disordered collagen to ordered collagen showed a decrease at the 10 kGy radiation level from 1.522 ± 0.025 preirradiation to 1.489 ± 0.024 postirradiation (p = 0.01); no other detectable changes in Raman biomarkers were observed.
Conclusion: The high-cycle fatigue life of cortical bone undergoes a nonlinear, dose-dependent decrease with an increase in gamma radiation sterilization in a clinically relevant dose range (0-25 kGy). Importantly, a notable drop-off in the high-cycle fatigue life of cortical bone appeared to occur between 17.5 kGy and 25 kGy, correlating to a sixfold decrease in mean cycles to failure. We speculate that the decrease in the Raman biomarker for disordered collagen at 10 kGy with no loss in high-cycle fatigue life may be caused by an increased amount of nonenzymatic crosslinking of the collagen backbone relative to collagen chain-scission (whereas the benefits of crosslinking may be outweighed by excess scission of the collagen backbone at higher radiation doses), but future studies will need to ascertain whether this in fact is the case.
Clinical relevance: Radiation sterilization at the industry standard of 25 kGy has a substantial negative impact on the high-cycle fatigue life of cortical bone. Given these findings, it is possible to provide a meaningful increase in the high-cycle fatigue life and improve the overall functional lifetime of cortical bone allografts by lowering the radiation-sterilization dose below 25 kGy. Future work on radiation-sterilization methods at these clinically relevant doses is warranted to aid in preserving the high cycle fatigue life of cortical bone allografts while maintaining sterility.
Copyright © 2022 by the Association of Bone and Joint Surgeons.
Conflict of interest statement
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Figures




Comment in
-
CORR Insights®: The High-cycle Fatigue Life of Cortical Bone Allografts Is Radiation Sterilization Dose-dependent: An In Vitro Study.Clin Orthop Relat Res. 2022 Jun 1;480(6):1220-1221. doi: 10.1097/CORR.0000000000002182. Epub 2022 Mar 17. Clin Orthop Relat Res. 2022. PMID: 35302541 Free PMC article. No abstract available.
References
-
- Aamodt A, Lund-Larsen J, Eine J, Andersen E, Benum P, Husby OS. In vivo measurements show tensile axial strain in the proximal lateral aspect of the human femur. J Orthop Res. 1997;15:927-931. - PubMed
-
- Akkus O, Adar F, Schaffler MB. Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone. 2004;34:443-453. - PubMed
-
- Akkus O, Belaney RM. Sterilization by gamma radiation impairs the tensile fatigue life of cortical bone by two orders of magnitude. J Orthop Res. 2005;23:1054-1058. - PubMed
-
- Akkus O, Rimnac CM. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19:927-934. - PubMed
-
- Anderson M, Keyak J, Skinner H. Compressive mechanical properties of human cancellous bone after gamma irradiation. J Bone Joint Surg Am. 1992;74:747-752. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

