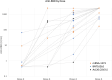
Improved Antibody Response After a Fifth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series
- PMID: 35175241
- PMCID: PMC9038246
- DOI: 10.1097/TP.0000000000004092
Improved Antibody Response After a Fifth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series
Abstract

Conflict of interest statement
D.L.S. received consulting and speaking honoraria from Sanofi, Novartis, CLS Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, Thermo Fisher Scientific, Regeneron, and AstraZeneca. M.L.L. is the Social Media Editor for Transplantation. R.K.A. has grant/research support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, and Takeda/Shire. The other authors declare no conflicts of interest.
Figures
Comment in
-
Humoral Response After Five Successive Doses of SARS-CoV-2 mRNA Vaccine in Kidney Transplant Patients.Transplantation. 2023 Jul 1;107(7):e188-e189. doi: 10.1097/TP.0000000000004628. Epub 2023 May 12. Transplantation. 2023. PMID: 37170412 No abstract available.
References
-
- Callaghan CJ, Mumford L, Curtis RMK, et al. . Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. [Epub ahead of print. January 4, 2022]. doi:10.1097/TP.0000000000004059 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous