Molecular Biomarker Testing for the Diagnosis of Diffuse Gliomas
- PMID: 35175291
- PMCID: PMC9311267
- DOI: 10.5858/arpa.2021-0295-CP
Molecular Biomarker Testing for the Diagnosis of Diffuse Gliomas
Abstract
Context.—: The diagnosis and clinical management of patients with diffuse gliomas (DGs) have evolved rapidly over the past decade with the emergence of molecular biomarkers that are used to classify, stratify risk, and predict treatment response for optimal clinical care.
Objective.—: To develop evidence-based recommendations for informing molecular biomarker testing for pediatric and adult patients with DGs and provide guidance for appropriate laboratory test and biomarker selection for optimal diagnosis, risk stratification, and prediction.
Design.—: The College of American Pathologists convened an expert panel to perform a systematic review of the literature and develop recommendations. A systematic review of literature was conducted to address the overarching question, "What ancillary tests are needed to classify DGs and sufficiently inform the clinical management of patients?" Recommendations were derived from quality of evidence, open comment feedback, and expert panel consensus.
Results.—: Thirteen recommendations and 3 good practice statements were established to guide pathologists and treating physicians on the most appropriate methods and molecular biomarkers to include in laboratory testing to inform clinical management of patients with DGs.
Conclusions.—: Evidence-based incorporation of laboratory results from molecular biomarker testing into integrated diagnoses of DGs provides reproducible and clinically meaningful information for patient management.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found in the Appendix at the end of this article.
Figures

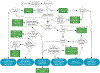
References
-
- Louis DN, Oghaki H, Wiestler OD, Caenee WK, eds. Who Classification of Tumours of the Central Nervous System. 4th ed. WHO Press; 2016. World Health Organization Classification of Tumours, vol 1.

