Proptosis and Diplopia Response With Teprotumumab and Placebo vs the Recommended Treatment Regimen With Intravenous Methylprednisolone in Moderate to Severe Thyroid Eye Disease: A Meta-analysis and Matching-Adjusted Indirect Comparison
- PMID: 35175308
- PMCID: PMC8855315
- DOI: 10.1001/jamaophthalmol.2021.6284
Proptosis and Diplopia Response With Teprotumumab and Placebo vs the Recommended Treatment Regimen With Intravenous Methylprednisolone in Moderate to Severe Thyroid Eye Disease: A Meta-analysis and Matching-Adjusted Indirect Comparison
Abstract
Importance: Thyroid eye disease can be a debilitating autoimmune disorder characterized by progressive proptosis or diplopia. Teprotumumab has been compared with placebo in randomized clinical trials, but not with intravenous methylprednisolone (IVMP), which sometimes is used in clinical practice for this condition.
Objective: To conduct a matching-adjusted indirect comparison of teprotumumab vs IVMP vs placebo.
Data sources: Deidentified patient-level data from teprotumumab trials and aggregate-level data from literature on the most recommended regimen of IVMP.
Study selection: PubMed and Embase were searched for randomized/observational studies using key terms and controlled vocabulary. Full texts of eligible articles were reviewed and cataloged.
Data extraction and synthesis: Conducted by 1 reviewer (R.A.Q.) and 1 verifier (R.B.), including study characteristics, eligibility criteria, baseline characteristics, and outcomes.
Main outcomes and measures: Changes in proptosis by millimeter and diplopia response (percentage with ≥1 grade reduction) from baseline to week 12 in patients receiving IVMP and placebo, and to week 24 in patients receiving teprotumumab.
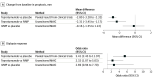
Results: The search identified 1019 records, and 6 through manual searches, alerts, and secondary references. After excluding duplicates and screening full-text records, 12 IVMP studies were included in the matching-adjusted indirect comparison (11 for proptosis change [n = 419], 4 for diplopia response [n = 125], and 2 teprotumumab [n = 79] and placebo [n = 83] comparator studies). Treatment with IVMP resulted in a proptosis difference of -0.16 mm (95% CI, -1.55 to 1.22 mm) from baseline to week 12 vs placebo. The proptosis treatment difference between IVMP and teprotumumab of -2.31 mm (95% CI, -3.45 to -1.17 mm) favored teprotumumab. Treatment with IVMP (odds ratio, 2.69; 95% CI, 0.94-7.70) was not favored over placebo in odds of diplopia response; however, teprotumumab was favored over IVMP (odds ratio, 2.32; 95% CI, 1.07-5.03).
Conclusions and relevance: This meta-analysis suggests that use of IVMP is associated with a small, typically not clinically relevant, change from baseline in proptosis vs placebo, with modest changes in diplopia. While this nonrandomized comparison suggests that use of teprotumumab, compared with IVMP, is associated with greater improvements in proptosis and may be twice as likely to have a 1 grade or higher reduction in diplopia, randomized trials comparing these 2 treatments would be warranted to determine if 1 treatment is superior to the other to a clinically relevant degree.
Conflict of interest statement
Figures

Comment in
-
Teprotumumab vs Intravenous Methylprednisolone for Thyroid Eye Disease: Is There Still a Role for Steroids?JAMA Ophthalmol. 2022 Apr 1;140(4):335-336. doi: 10.1001/jamaophthalmol.2021.6376. JAMA Ophthalmol. 2022. PMID: 35175298 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

