Effect of Capecitabine Maintenance Therapy Plus Best Supportive Care vs Best Supportive Care Alone on Progression-Free Survival Among Patients With Newly Diagnosed Metastatic Nasopharyngeal Carcinoma Who Had Received Induction Chemotherapy: A Phase 3 Randomized Clinical Trial
- PMID: 35175316
- PMCID: PMC8855317
- DOI: 10.1001/jamaoncol.2021.7366
Effect of Capecitabine Maintenance Therapy Plus Best Supportive Care vs Best Supportive Care Alone on Progression-Free Survival Among Patients With Newly Diagnosed Metastatic Nasopharyngeal Carcinoma Who Had Received Induction Chemotherapy: A Phase 3 Randomized Clinical Trial
Abstract
Importance: Capecitabine maintenance therapy improves survival outcomes in various cancer types, but data are limited on the efficacy and safety of capecitabine maintenance therapy in metastatic nasopharyngeal carcinoma (NPC).
Objective: To investigate the efficacy and safety of capecitabine maintenance therapy in metastatic NPC.
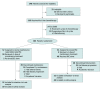
Design, setting, and participants: This randomized phase 3 clinical trial was conducted at Sun Yat-sen University Cancer Center from May 16, 2015, to January 9, 2020, among 104 patients with newly diagnosed metastatic NPC who had achieved disease control after 4 to 6 cycles of induction chemotherapy with paclitaxel, cisplatin, and capecitabine. The final follow-up date was May 30, 2021. All efficacy analyses were conducted in the intention-to-treat population.
Interventions: Eligible patients were randomly assigned (1:1) to receive either capecitabine maintenance therapy (1000 mg/m2 orally twice daily on days 1-14) every 3 weeks plus best supportive care (BSC) (capecitabine maintenance group) or BSC alone after 4 to 6 cycles of induction chemotherapy.
Main outcomes and measures: Progression-free survival (PFS). Secondary end points were objective response rate, duration of response, overall survival, and safety.
Results: This study included 104 patients (84 men [80.8%]; median age, 47 years [IQR, 38-54 years]), with 52 assigned to the capecitabine maintenance group and 52 assigned to the BSC group. After a median follow-up of 33.8 months (IQR, 22.9-50.7 months), there were 23 events (44.2%) of progression or death in the capecitabine maintenance group and 37 events (71.2%) of progression or death in the BSC group. Median PFS survival was significantly higher in the capecitabine maintenance group (35.9 months [95% CI, 20.5 months-not reached]) than in the BSC group (8.2 months [95% CI, 6.4-10.0 months]), with a hazard ratio of 0.44 (95% CI, 0.26-0.74; P = .002). Higher objective response rates and longer median duration of response were observed in the capecitabine maintenance group (25.0%; 40.0 months) compared with the BSC group (objective response rate, 25.0% [n = 13] vs 11.5% [n = 6]; and median duration of response, 40.0 months [95% CI, not reached-not reached] vs 13.2 months [95% CI, 9.9-16.5 months]). The most common grade 3 or 4 adverse events during maintenance therapy were anemia (6 of 50 [12.0%]), hand-foot syndrome (5 of 50 [10.0%]), nausea and vomiting (3 of 50 [6.0%]), fatigue (2 of 50 [4.0%]), and mucositis (2 of 50 [4.0%]). No deaths in the maintenance group were deemed treatment-related.
Conclusions and relevance: In this phase 3 randomized clinical trial, capecitabine maintenance therapy significantly improved PFS for patients with newly diagnosed metastatic NPC who achieved disease control after capecitabine-containing induction chemotherapy. Capecitabine exhibited manageable toxic effects.
Trial registration: ClinicalTrials.gov Identifier: NCT02460419.
Conflict of interest statement
Figures


Comment in
-
Maintenance Capecitabine in Recurrent or Metastatic Nasopharyngeal Carcinoma-Magic Bullet or Pandora's Box?JAMA Oncol. 2022 Apr 1;8(4):524-525. doi: 10.1001/jamaoncol.2021.7365. JAMA Oncol. 2022. PMID: 35175303 No abstract available.
-
Maintenance capecitabine is beneficial in NPC.Nat Rev Clin Oncol. 2022 Apr;19(4):220. doi: 10.1038/s41571-022-00618-0. Nat Rev Clin Oncol. 2022. PMID: 35256771 No abstract available.
-
Capecitabine Maintenance in Metastatic Nasopharyngeal Carcinoma-Reply.JAMA Oncol. 2022 Aug 1;8(8):1224-1225. doi: 10.1001/jamaoncol.2022.2068. JAMA Oncol. 2022. PMID: 35771532 No abstract available.
-
Capecitabine Maintenance in Metastatic Nasopharyngeal Carcinoma.JAMA Oncol. 2022 Aug 1;8(8):1223-1224. doi: 10.1001/jamaoncol.2022.2062. JAMA Oncol. 2022. PMID: 35771541 No abstract available.
-
Capecitabine Maintenance in Metastatic Nasopharyngeal Carcinoma.JAMA Oncol. 2022 Aug 1;8(8):1224. doi: 10.1001/jamaoncol.2022.2065. JAMA Oncol. 2022. PMID: 35771563 No abstract available.
References
-
- National Comprehensive Cancer Network . Head and neck cancers, version 1.2015. Accessed May 12, 2015. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.2015 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

