Bruton's tyrosine kinase inhibition-An emerging therapeutic strategy in immune-mediated dermatological conditions
- PMID: 35175630
- PMCID: PMC9545595
- DOI: 10.1111/all.15261
Bruton's tyrosine kinase inhibition-An emerging therapeutic strategy in immune-mediated dermatological conditions
Abstract
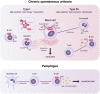
Bruton's tyrosine kinase (BTK), a member of the Tec kinase family, is critically involved in a range of immunological pathways. The clinical application of BTK inhibitors for B-cell malignancies has proven successful, and there is strong rationale for the potential benefits of BTK inhibitors in some autoimmune and allergic conditions, including immune-mediated dermatological diseases. However, the established risk-to-benefit profile of "first-generation" BTK inhibitors cannot be extrapolated to these emerging, non-oncological, indications. "Next-generation" BTK inhibitors such as remibrutinib and fenebrutinib entered clinical development for chronic spontaneous urticaria (CSU); rilzabrutinib and tirabrutinib are being studied as potential treatments for pemphigus. Promising data from early-phase clinical trials in CSU suggest potential for these agents to achieve strong pathway inhibition, which may translate into measurable clinical benefits, as well as other effects such as the disruption of autoantibody production. BTK inhibitors may help to overcome some of the shortcomings of monoclonal antibody treatments for immune-mediated dermatological conditions such as CSU, pemphigus, and systemic lupus erythematosus. In addition, the use of BTK inhibitors may improve understanding of the pathophysiological roles of mast cells, basophils, and B cells in such conditions.
Keywords: Bruton's tyrosine kinase inhibitor; chronic spontaneous urticaria; fenebrutinib; pemphigus; remibrutinib.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
PM‐B received honoraria for acting as a consultant and/or as a speaker for AbbVie, Janssen, Novartis, LEO Pharma, Almirall, Sanofi, Viatris, L’Oréal, and Cantabria Labs and served as a principal investigator in clinical trials supported by AbbVie, Sanofi, and Novartis. AB received honoraria for lectures and educational events for LEO Pharma, Janssen‐Cilag, AbbVie, and Novartis. PK received payment/honoraria for lectures/presentations outside of submitted work for Novartis and Roche.. SM‐R received funding from GA²LEN Global Allergy and Asthma European Network. MM served as a speaker and/or advisor for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Blueprint, Celldex, Centogene, CSL Behring, FAES, Genentech, Gilead, GIInnovation, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third Harmonic Bio, UCB, and Uriach. SF and JS have no conflicts of interest to disclose.
Figures

References
-
- Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9(6):722‐728. - PubMed
-
- Vetrie D, Vořechovský I, Sideras P, et al. The gene involved in X‐linked agammaglobulinaemia is a member of the src family of protein‐tyrosine kinases. Nature. 1993;361(6409):226‐233. - PubMed
-
- Tsukada S, Saffran DC, Rawlings DJ, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X‐linked agammaglobulinemia. Cell. 1993;72(2):279‐290. - PubMed
-
- Thomas JD, Sideras P, Smith CI, Vorechovsky I, Chapman V, Paul WE. Colocalization of X‐linked agammaglobulinemia and X‐linked immunodeficiency genes. Science. 1993;261(5119):355‐358. - PubMed
-
- Rawlings DJ, Saffran DC, Tsukada S, et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261(5119):358‐361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

