Potential effect of tolvaptan on polycystic liver disease for patients with ADPKD meeting the Japanese criteria of tolvaptan use
- PMID: 35176098
- PMCID: PMC8853523
- DOI: 10.1371/journal.pone.0264065
Potential effect of tolvaptan on polycystic liver disease for patients with ADPKD meeting the Japanese criteria of tolvaptan use
Abstract
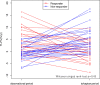
Polycystic liver disease (PLD) is a common extrarenal complication of autosomal dominant polycystic kidney disease (ADPKD), which causes compression-related syndrome and ultimately leads to liver dysfunction. Tolvaptan, a V2 receptor antagonist, is widely used to protect kidney function in ADPKD but its effect on PLD remains unknown. An observational cohort study was conducted to evaluate tolvaptan's effect on patients with PLD due to ADPKD. After screening 902 patients, we found the 107 ADPKD patients with PLD who met the criteria of tolvaptan use in Japan. Among them, tolvaptan was prescribed for 62 patients (tolvaptan group), while the other was defined as the non-tolvaptan group. Compared with the non-tolvaptan group, the tolvaptan group had larger height-adjusted total kidney volume (median 994(range 450-4152) mL/m, 513 (405-1928) mL/m, p = 0.01), lower albumin level (mean 3.9±SD 0.4 g/dL, 4.3±0.4g/dL, p<0.01), and higher serum creatinine level (1.2±0.4 mg/dL, 0.9±0.2 mg/dL, p<0.01). Although the median change in annual growth rate of total liver volume (TLV) was not statistically different between the tolvaptan group (-0.8 (-15.9, 16.7) %/year) and the non-tolvaptan group (1.7 (-15.6-18.7) %/year)(p = 0.52), 20 (43.5%) patients in the tolvaptan group experienced a decrease in the growth rate of TLV (responders). A multivariable logistic regression model adjusting for related variables showed that older age (odds ratio 1.15 [95% CI 1.01-1.32]) and a higher growth rate of TLV in the non-tolvaptan period (odds 1.45 95% CI 1.10-1.90) were significantly associated with responders. In conclusion, the change in annual growth rate of TLV in ADPKD patients taking tolvaptan was not statistically different compared with that in ADPKD patients without taking tolvaptan. However, tolvaptan may have the potential to suppress the growth rate of TLV in some PLD patients due to ADPKD, especially in older patients or those that are rapid progressors of PLD. Several limitations were included in this study, therefore well-designed prospective studies were required to confirm the effect of tolvaptan on PLD.
Conflict of interest statement
This work was supported by a commercial source, J.H’s competitive research grant from Otsuka Pharm, Japan. This commercial funder, however, did not relate to employment, consultancy, patents, products in development, marketed product nor had influenced study design, data collection and analysis, decision to publish, or preparation of the manuscript. In addition, this funder does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Tolvaptan in Japanese patients with later-stage autosomal dominant polycystic kidney disease.J Nephrol. 2018 Dec;31(6):961-966. doi: 10.1007/s40620-018-0545-8. Epub 2018 Oct 24. J Nephrol. 2018. PMID: 30357715
-
Urinary Lithogenic Risk Profile in ADPKD Patients Treated with Tolvaptan.Clin J Am Soc Nephrol. 2020 Jul 1;15(7):1007-1014. doi: 10.2215/CJN.13861119. Epub 2020 Jun 11. Clin J Am Soc Nephrol. 2020. PMID: 32527945 Free PMC article.
-
Effect of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease by CKD Stage: Results from the TEMPO 3:4 Trial.Clin J Am Soc Nephrol. 2016 May 6;11(5):803-811. doi: 10.2215/CJN.06300615. Epub 2016 Feb 23. Clin J Am Soc Nephrol. 2016. PMID: 26912543 Free PMC article. Clinical Trial.
-
Autosomal dominant polycystic kidney disease: an overview of recent genetic and clinical advances.Ren Fail. 2025 Dec;47(1):2492374. doi: 10.1080/0886022X.2025.2492374. Epub 2025 Apr 23. Ren Fail. 2025. PMID: 40268755 Free PMC article. Review.
-
An update on tolvaptan for autosomal dominant polycystic kidney disease.Drugs Today (Barc). 2018 Sep;54(9):519-533. doi: 10.1358/dot.2018.54.9.2776624. Drugs Today (Barc). 2018. PMID: 30303493 Review.
Cited by
-
Brachial-ankle pulse wave velocity predicts liver volume in patients with autosomal dominant polycystic kidney disease.PLoS One. 2025 Jul 21;20(7):e0328133. doi: 10.1371/journal.pone.0328133. eCollection 2025. PLoS One. 2025. PMID: 40690501 Free PMC article.
-
Autosomal Dominant Polycystic Kidney Disease: Extrarenal Involvement.Int J Mol Sci. 2024 Feb 22;25(5):2554. doi: 10.3390/ijms25052554. Int J Mol Sci. 2024. PMID: 38473800 Free PMC article. Review.
References
-
- Cornec-Le Gall E, Blais JD, Irazabal MV, Devuyst O, Gansevoort RT, Perrone RD, et al.. Can we further enrich autosomal dominant polycystic kidney disease clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial. Nephrol Dial Transplant 2018; 33(4): 645–652. Epub 2018/4/13. doi: 10.1093/ndt/gfx188 . PMCID: PMC5888998. - DOI - PMC - PubMed
-
- Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, et al.. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 2006; 1(1): 64–9. Epub 2005/10/26. doi: 10.2215/CJN.00080605 . - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

