Spontaneous Membranization in a Silk-Based Coacervate Protocell Model
- PMID: 35176203
- PMCID: PMC9306657
- DOI: 10.1002/anie.202202302
Spontaneous Membranization in a Silk-Based Coacervate Protocell Model
Abstract
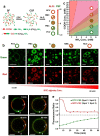
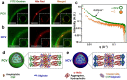
Molecularly crowded coacervate micro-droplets are useful protocell constructs but the absence of a physical membrane limits their application as cytomimetic models. Auxiliary surface-active agents have been harnessed to stabilize the coacervate droplets by irreversible shell formation but endogenous processes of reversible membranization have received minimal attention. Herein, we describe a dynamic alginate/silk coacervate-based protocell model in which membrane-less droplets are reversibly reconfigured and inflated into semipermeable coacervate vesicles by spontaneous self-organization of amphiphilic silk polymers at the droplet surface under non-neutral charge conditions in the absence of auxiliary agents. We show that membranization can be reversibly controlled endogenously by programming the pH within the protocells using an antagonistic enzyme system such that structural reconfigurations in the protocell microstructure are coupled to the trafficking of water-soluble solutes. Our results open new perspectives in the design of hybrid protocell models with dynamical structural properties.
Keywords: Coacervates; Protocells; Silk.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

