Zafirlukast inhibits the growth of lung adenocarcinoma via inhibiting TMEM16A channel activity
- PMID: 35176281
- PMCID: PMC8931426
- DOI: 10.1016/j.jbc.2022.101731
Zafirlukast inhibits the growth of lung adenocarcinoma via inhibiting TMEM16A channel activity
Abstract
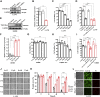
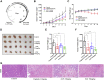
Lung cancer has the highest mortality among cancers worldwide due to its high incidence and lack of the effective cures. We have previously demonstrated that the membrane ion channel TMEM16A is a potential drug target for the treatment of lung adenocarcinoma and have identified a pocket of inhibitor binding that provides the basis for screening promising new inhibitors. However, conventional drug discovery strategies are lengthy and costly, and the unpredictable side effects lead to a high failure rate in drug development. Therefore, finding new therapeutic directions for already marketed drugs may be a feasible strategy to obtain safe and effective therapeutic drugs. Here, we screened a library of over 1400 Food and Drug Administration-approved drugs through virtual screening and activity testing. We identified a drug candidate, Zafirlukast (ZAF), clinically approved for the treatment of asthma, that could inhibit the TMEM16A channel in a concentration-dependent manner. Molecular dynamics simulations and site-directed mutagenesis experiments showed that ZAF can bind to S387/N533/R535 in the nonselective inhibitor binding pocket, thereby blocking the channel pore. Furthermore, we demonstrate ZAF can target TMEM16A channel to inhibit the proliferation and migration of lung adenocarcinoma LA795 cells. In vivo experiments showed that ZAF can significantly inhibit lung adenocarcinoma tumor growth in mice. Taken together, we identified ZAF as a novel TMEM16A channel inhibitor with excellent anticancer activity, and as such, it represents a promising candidate for future preclinical and clinical studies.
Keywords: TMEM16A; inhibitor; ion channel; zafirlukast.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interests.
Figures




References
-
- Stewart B., Wild C. International Agency for Research on Cancer; Lyon, France: 2014. World Cancer Report 2014.
-
- Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021;149:778–789. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

