COVID-19 in dialysis: clinical impact, immune response, prevention, and treatment
- PMID: 35176326
- PMCID: PMC8842412
- DOI: 10.1016/j.kint.2022.01.022
COVID-19 in dialysis: clinical impact, immune response, prevention, and treatment
Abstract
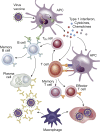
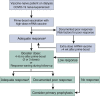
The COVID-19 pandemic has profound adverse effects on the population on dialysis. Patients requiring dialysis are at an increased risk of SARS-CoV-2 infection and mortality, and many have experienced psychological distress as well as delayed or suboptimal care. COVID-19 survivors have prolonged viral shedding, but generally develop a robust and long-lasting humoral immune response that correlates with initial disease severity. However, protection against reinfection is incomplete. A growing body of evidence reveals delayed and blunted immune responses to SARS-CoV-2 vaccination. Administration of a third dose within 1 to 2 months of prime-boost vaccination significantly increases antibody levels, in particular in patients with poor initial responses. Patients on dialysis have inferior immune responses to adenoviral vector vaccines than to mRNA vaccines. The immunogenicity of the mRNA-1273 vaccine is markedly better than that of the BNT162b2 vaccine, most likely by virtue of its higher mRNA content. Despite suboptimal immune responses in patients on dialysis, preliminary data suggest that vaccination partially protects against infection and severe disease requiring hospitalization. However, progressive waning of immunity and emergence of SARS-CoV-2 variants with a high potential of immune escape call for a booster dose in all patients on dialysis 4 to 6 months after prime-boost vaccination. Patients with persistent poor vaccine responses may be candidates for primary prophylaxis strategies. In the absence of specific data in patients on dialysis, therapeutic strategies in the event of established COVID-19 must be extrapolated from evidence obtained in the population not on dialysis. Neutralizing monoclonal antibodies may be an attractive option after a high-risk exposure or during the early course of infection.
Keywords: COVID-19; SARS-CoV-2; dialysis; hemodialysis; immune response; treatment; vaccination.
Copyright © 2022 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

