Palmitoylethanolamide dampens neuroinflammation and anxiety-like behavior in obese mice
- PMID: 35176443
- PMCID: PMC10662208
- DOI: 10.1016/j.bbi.2022.02.008
Palmitoylethanolamide dampens neuroinflammation and anxiety-like behavior in obese mice
Abstract
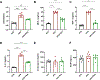
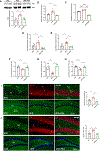
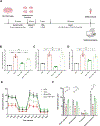
High-fat diet (HFD) consumption leads to obesity and a chronic state of low-grade inflammation, named metainflammation. Notably, metainflammation contributes to neuroinflammation due to the increased levels of circulating free fatty acids and cytokines. It indicates a strict interplay between peripheral and central counterparts in the pathogenic mechanisms of obesity-related mood disorders. In this context, the impairment of internal hypothalamic circuitry runs in tandem with the alteration of other brain areas associated with emotional processing (i.e., hippocampus and amygdala). Palmitoylethanolamide (PEA), an endogenous lipid mediator belonging to the N-acylethanolamines family, has been extensively studied for its pleiotropic effects both at central and peripheral level. Our study aimed to elucidate PEA capability in limiting obesity-induced anxiety-like behavior and neuroinflammation-related features in an experimental model of HFD-fed obese mice. PEA treatment promoted an improvement in anxiety-like behavior of obese mice and the systemic inflammation, reducing serum pro-inflammatory mediators (i.e., TNF-α, IL-1β, MCP-1, LPS). In the amygdala, PEA increased dopamine turnover, as well as GABA levels. PEA also counteracted the overactivation of HPA axis, reducing the expression of hypothalamic corticotropin-releasing hormone and its type 1 receptor. Moreover, PEA attenuated the immunoreactivity of Iba-1 and GFAP and reduced pro-inflammatory pathways and cytokine production in both the hypothalamus and hippocampus. This finding, together with the reduced transcription of mast cell markers (chymase 1 and tryptase β2) in the hippocampus, indicated the weakening of immune cell activation underlying the neuroprotective effect of PEA. Obesity-driven neuroinflammation was also associated with the disruption of blood-brain barrier (BBB) in the hippocampus. PEA limited the albumin extravasation and restored tight junction transcription modified by HFD. To gain mechanistic insight, we designed an in vitro model of metabolic injury using human neuroblastoma SH-SY5Y cells insulted by a mix of glucosamine and glucose. Here, PEA directly counteracted inflammation and mitochondrial dysfunction in a PPAR-α-dependent manner since the pharmacological blockade of the receptor reverted its effects. Our results strengthen the therapeutic potential of PEA in obesity-related neuropsychiatric comorbidities, controlling neuroinflammation, BBB disruption, and neurotransmitter imbalance involved in behavioral dysfunctions.
Keywords: Astrogliosis; Blood–brain barrier permeability; High-fat diet; Inflammation; Mastocytosis; Metabolic impairment; Microgliosis; Mood disorders; N-acylethanolamines; Peroxisome proliferator-activated receptor-α.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Annunziata C, Lama A, Pirozzi C, Cavaliere G, Trinchese G, Di Guida F, Nitrato Izzo A, Cimmino F, Paciello O, De Biase D, Murru E, Banni S, Calignano A, Mollica MP, Mattace Raso G, Meli R, 2020. Palmitoylethanolamide counteracts hepatic metabolic inflexibility modulating mitochondrial function and efficiency in diet-induced obese mice. FASEB J. 34 (1), 350–364. 10.1096/fj.201901510RR. - DOI - PubMed
-
- Artamonov M, Zhukov O, Shuba I, Storozhuk L, Khmel T, Klimashevsky V, Mikosha A, Gula N, 2005. Incorporation of labelled N-acylethanolamine (NAE) into rat brain regions in vivo and adaptive properties of saturated NAE under x-ray irradiation. Ukr. Biokhim. Zh. 1999 (77), 51–62. - PubMed
-
- Beggiato Sarah, Tomasini Maria Cristina, Cassano Tommaso, Ferraro Luca, 2020. Chronic oral palmitoylethanolamide administration rescues cognitive deficit and reduces neuroinflammation, oxidative stress, and glutamate levels in a transgenic murine model of Alzheimer’s Disease. Journal of Clinical Medicine 9(2), 428. 10.3390/jcm9020428. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

