Drug-Induced Hypersensitivity Syndrome (DIHS)/Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS): Clinical Features and Pathogenesis
- PMID: 35176506
- PMCID: PMC9201940
- DOI: 10.1016/j.jaip.2022.02.004
Drug-Induced Hypersensitivity Syndrome (DIHS)/Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS): Clinical Features and Pathogenesis
Abstract
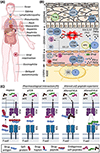
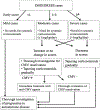
Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS) is one example of a severe delayed T-cell-mediated adverse drug reaction. DIHS/DRESS presents with fever, widespread rash and facial edema, organ involvement, and hematological abnormalities, including eosinophilia and atypical lymphocytosis. DIHS/DRESS is associated with relapse 2 to 4 weeks after acute symptoms, often coinciding with reactivation of prevalent chronic persistent human herpesviruses such as human herpesvirus 6, EBV, and cytomegalovirus. The mortality of DIHS/DRESS is up to 10% and often related to unrecognized myocarditis and cytomegalovirus complications, with longer-term consequences that contribute to morbidity including autoimmune diseases such as thyroiditis. It is essential that all potential drug causes, including all new drugs introduced within the 8 weeks preceding onset of DIHS/DRESS symptoms, are identified. All potential drug culprits, as well as drugs that are closely related structurally to the culprit drug, should be avoided in the future. Systemic corticosteroids have remained the mainstay for the treatment of DIHS/DRESS with internal organ involvement. Steroid-sparing agents, such as cyclosporine, mycophenolate mofetil, and monthly intravenous immune globulin, have been successfully used for treatment, and careful follow-up for cytomegalovirus reactivation is recommended. Strong associations between HLA class I alleles and DIHS/DRESS predisposition include HLA-B∗13:01 and dapsone, HLA-B∗58:01 and allopurinol, and HLA-B∗32:01 and vancomycin. These have opened a pathway for prevention, risk stratification, and earlier diagnosis. Single-cell sequencing and other studies of immunopathogenesis promise to identify targeted treatment approaches.
Keywords: Autoimmune; CMV; Corticosteroids; DIHS; DRESS; EBV; Eosinophilia; HHV-6; HLA; Hypersensitivity.
Copyright © 2022 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Chaiken BH, Goldberg BI, Segal JP. Dilantin sensitivity: report of a case of hepatitis with jaundice, pyrexia and exfoliative dermatitis. N Engl J Med 1950; 242:897–8. - PubMed
-
- Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical fea-tures and viral reactivations. Br J Dermatol 2007;156:1083–4. - PubMed
-
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol 2007;156:609–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

