Melatonin as a Chronobiotic with Sleep-promoting Properties
- PMID: 35176989
- PMCID: PMC10227911
- DOI: 10.2174/1570159X20666220217152617
Melatonin as a Chronobiotic with Sleep-promoting Properties
Abstract
The use of exogenous melatonin (exo-MEL) as a sleep-promoting drug has been under extensive debate due to the lack of consistency of its described effects. In this study, we conduct a systematic and comprehensive review of the literature on the chronobiotic, sleep-inducing, and overall sleep-promoting properties of exo-MEL. To this aim, we first describe the possible pharmacological mechanisms involved in the sleep-promoting properties and then report the corresponding effects of exo-MEL administration on clinical outcomes in: a) healthy subjects, b) circadian rhythm sleep disorders, c) primary insomnia. Timing of administration and doses of exo-MEL received particular attention in this work. The exo-MEL pharmacological effects are hereby interpreted in view of changes in the physiological properties and rhythmicity of endogenous melatonin. Finally, we discuss some translational implications for the personalized use of exo-MEL in the clinical practice.
Keywords: Melatonin; chronobiotic; circadian rhythm sleep disorders; hypnotic; insomnia; sleep-inducing; sleep-promoting; soporific.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
U.F. is co-founder and president of sleepActa S.r.l, a spin-off company of the University of Pisa operating in the field of sleep medicine. M.S. has been a consultant for Pierpaoli Exelyas Srl. All other authors declare that this article content has no conflict of interest.
Figures
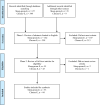

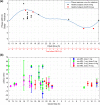
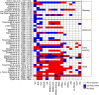
References
-
- Lerner A.B., Case J.D., Takahashi Y., Lee T.H., Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes1. J. Am. Chem. Soc. 1958;80(10):2587. doi: 10.1021/ja01543a060. - DOI
-
- Lerner Aaron B. CJD. Melatonin. Intersociey Symp New Negleted Horm; 1962. pp. 590–592.
-
- Vollrath L., Semm P., Gammel G. Sleep induction by intranasal application of melatonin. Bremen, FRG: Symp. Melatonin; 1981. pp. 327–329. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

