A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D-a randomised multicentre international clinical trial
- PMID: 35177066
- PMCID: PMC8853840
- DOI: 10.1186/s12916-022-02290-8
A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D-a randomised multicentre international clinical trial
Abstract
Background: Vitamin D status has been implicated in COVID-19 disease. The objective of the COVID-VIT-D trial was to investigate if an oral bolus of cholecalciferol (100,000 IU) administered at hospital admission influences the outcomes of moderate-severe COVID-19 disease. In the same cohort, the association between baseline serum calcidiol levels with the same outcomes was also analysed.
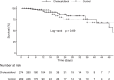
Methods: The COVID-VIT-D is a multicentre, international, randomised, open label, clinical trial conducted throughout 1 year. Patients older than 18 years with moderate-severe COVID-19 disease requiring hospitalisation were included. At admission, patients were randomised 1:1 to receive a single oral bolus of cholecalciferol (n=274) or nothing (n=269). Patients were followed from admission to discharge or death. Length of hospitalisation, admission to intensive care unit (ICU) and mortality were assessed.
Results: In the randomised trial, comorbidities, biomarkers, symptoms and drugs used did not differ between groups. Median serum calcidiol in the cholecalciferol and control groups were 17.0 vs. 16.1 ng/mL at admission and 29.0 vs. 16.4 ng/mL at discharge, respectively. The median length of hospitalisation (10.0 [95%CI 9.0-10.5] vs. 9.5 [95%CI 9.0-10.5] days), admission to ICU (17.2% [95%CI 13.0-22.3] vs. 16.4% [95%CI 12.3-21.4]) and death rate (8.0% [95%CI 5.2-12.1] vs. 5.6% [95%CI 3.3-9.2]) did not differ between the cholecalciferol and control group. In the cohort analyses, the highest serum calcidiol category at admission (>25ng/mL) was associated with lower percentage of pulmonary involvement and better outcomes.
Conclusions: The randomised clinical trial showed the administration of an oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve the outcomes of the COVID-19 disease. A cohort analysis showed that serum calcidiol at hospital admission was associated with outcomes.
Trial registration: COVID-VIT-D trial was authorised by the Spanish Agency for Medicines and Health products (AEMPS) and registered in European Union Drug Regulating Authorities Clinical Trials (EudraCT 2020-002274-28) and in ClinicalTrials.gov ( NCT04552951 ).
Keywords: COVID-19 disease; Cholecalciferol; SARS-CoV-2; Vitamin D.
© 2022. The Author(s).
Conflict of interest statement
The following authors received research grants fees, grants for congress attending, courses and collaborations by the following entities: Jorge B. Cannata-Andía from Amgen, Kyowa-Kirim and Vifor Pharma; Ricardo Mouzo from Takeda, Otsuka, Nipro, Sanofi-Aventis, Amgen and the Senefro Foundation; Natalia Carrillo-López from Ministerio de Ciencia e Innovación (MICINN)/Instituto de Salud Carlos III (ISCIII); Sara Panizo from MICINN/ISCIII and Luis Hernando gran from Fundación Renal Íñigo Álvarez de Toledo; Carolina Ballarino from Pfizer, Takeda and Sanofi-Aventis; Jacqueline Pefaur-Penna from Novartis and Sanofi-Aventis; Jesús Calviño-Varela from Baxter, Otsuka, Palex, Astra, Vifor and Chiesi; Carlos Gómez-Alonso from Amgen, UCB, Stada, Grünenthal, Gebro Pharma, FAES, Kiowa-Kirin and Laboratorios Rubió; John Cunningham from Amgen, Merck and Vifor Pharma; Manuel Naves-Díaz from MICINN/ISCIII, Amgen, UCB, Kyowa-Kirim, Stada, Italfármaco, Gebro Pharma, Rubió, Gedeon Richter, Grünenthal and FEIOMM and José L. Fernández-Martín from MICINN/ISCIII. The rest of authors are not aware of any additional relationship, funding or financial holdings.
Figures




References
-
- Holick MF, Vitamin D. Physiology, molecular biology, and clinical applications: Totowa. NJ: Humana Press; 2010.
-
- Illescas-Montes R, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ. Vitamin D and autoimmune diseases. Life Sci. 2019;233:116744. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

