The etiology of preeclampsia
- PMID: 35177222
- PMCID: PMC8988238
- DOI: 10.1016/j.ajog.2021.11.1356
The etiology of preeclampsia
Abstract
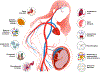
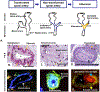
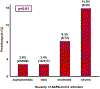
Preeclampsia is one of the "great obstetrical syndromes" in which multiple and sometimes overlapping pathologic processes activate a common pathway consisting of endothelial cell activation, intravascular inflammation, and syncytiotrophoblast stress. This article reviews the potential etiologies of preeclampsia. The role of uteroplacental ischemia is well-established on the basis of a solid body of clinical and experimental evidence. A causal role for microorganisms has gained recognition through the realization that periodontal disease and maternal gut dysbiosis are linked to atherosclerosis, thus possibly to a subset of patients with preeclampsia. The recent reports indicating that SARS-CoV-2 infection might be causally linked to preeclampsia are reviewed along with the potential mechanisms involved. Particular etiologic factors, such as the breakdown of maternal-fetal immune tolerance (thought to account for the excess of preeclampsia in primipaternity and egg donation), may operate, in part, through uteroplacental ischemia, whereas other factors such as placental aging may operate largely through syncytiotrophoblast stress. This article also examines the association between gestational diabetes mellitus and maternal obesity with preeclampsia. The role of autoimmunity, fetal diseases, and endocrine disorders is discussed. A greater understanding of the etiologic factors of preeclampsia is essential to improve treatment and prevention.
Keywords: Ballantyne syndrome; COVID-19; Cushing’s syndrome; SARS-CoV-2; angiotensin receptor II; atherosclerosis; autoantibodies; body mass index; endothelial cell dysfunction; genetic incompatibility; gestational diabetes mellitus; hydatidiform mole; hydrops fetalis; hyperaldosteronism; hyperparathyroidism; hypertension; infection; inflammation; insulin resistance; intestinal dysbiosis; maternal antifetal rejection; metabolic syndrome; mirror syndrome; molar pregnancy; obesity; placental aging; placental ischemia; placental lesions of maternal vascular malperfusion; primipaternity; proteinuria; sleep disorders; sleep-disordered breathing; snoring; tolerance.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Figures
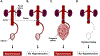





References
-
- Ogden E, Hildebrand G, Page EW. Rise of blood pressure during ischemia of the gravid uterus. Proc Soc Exp Biol Med 1940;43:49–51.
-
- Kumar D Chronic placental ischemia in relation to toxemias of pregnancy: a preliminary report. Am J Obstet Gynecol 1962;84:1323–29.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

