Early identification of patients admitted to hospital for covid-19 at risk of clinical deterioration: model development and multisite external validation study
- PMID: 35177406
- PMCID: PMC8850910
- DOI: 10.1136/bmj-2021-068576
Early identification of patients admitted to hospital for covid-19 at risk of clinical deterioration: model development and multisite external validation study
Abstract
Objective: To create and validate a simple and transferable machine learning model from electronic health record data to accurately predict clinical deterioration in patients with covid-19 across institutions, through use of a novel paradigm for model development and code sharing.
Design: Retrospective cohort study.
Setting: One US hospital during 2015-21 was used for model training and internal validation. External validation was conducted on patients admitted to hospital with covid-19 at 12 other US medical centers during 2020-21.
Participants: 33 119 adults (≥18 years) admitted to hospital with respiratory distress or covid-19.
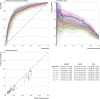
Main outcome measures: An ensemble of linear models was trained on the development cohort to predict a composite outcome of clinical deterioration within the first five days of hospital admission, defined as in-hospital mortality or any of three treatments indicating severe illness: mechanical ventilation, heated high flow nasal cannula, or intravenous vasopressors. The model was based on nine clinical and personal characteristic variables selected from 2686 variables available in the electronic health record. Internal and external validation performance was measured using the area under the receiver operating characteristic curve (AUROC) and the expected calibration error-the difference between predicted risk and actual risk. Potential bed day savings were estimated by calculating how many bed days hospitals could save per patient if low risk patients identified by the model were discharged early.
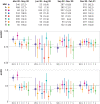
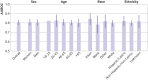
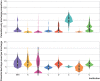
Results: 9291 covid-19 related hospital admissions at 13 medical centers were used for model validation, of which 1510 (16.3%) were related to the primary outcome. When the model was applied to the internal validation cohort, it achieved an AUROC of 0.80 (95% confidence interval 0.77 to 0.84) and an expected calibration error of 0.01 (95% confidence interval 0.00 to 0.02). Performance was consistent when validated in the 12 external medical centers (AUROC range 0.77-0.84), across subgroups of sex, age, race, and ethnicity (AUROC range 0.78-0.84), and across quarters (AUROC range 0.73-0.83). Using the model to triage low risk patients could potentially save up to 7.8 bed days per patient resulting from early discharge.
Conclusion: A model to predict clinical deterioration was developed rapidly in response to the covid-19 pandemic at a single hospital, was applied externally without the sharing of data, and performed well across multiple medical centers, patient subgroups, and time periods, showing its potential as a tool for use in optimizing healthcare resources.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from National Science Foundation (NSF), National Institutes of Health (NIH) -National Library of Medicine (NLM) and -National Heart, Lung, and Blood Institute (NHLBI), Agency for Healthcare Research and Quality (AHRQ), Centers for Disease Control and Prevention (CDC) -National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Precision Health at the University of Michigan, and the Institute for Healthcare Policy and Innovation at the University of Michigan. JZA received grant funding from National Institute on Aging, Michigan Department of Health and Human Services, and Merck Foundation, outside of the submitted work; JZA also received personal fees for consulting at JAMA Network and New England Journal of Medicine, honorariums from Harvard University, University of Chicago, and University of California San Diego, and monetary support for travel reimbursements from NIH, National Academy of Medicine, and AcademyHealth, during the conduct of the study; JZA also served as a board member of AcademyHealth, Physicians Health Plan, and Center for Health Research and Transformation, with no compensation, during the conduct of the study. SB reports receiving grant funding from NIH, outside of the submitted work. JPD reports receiving personal fees from the Annals of Emergency Medicine, during the conduct of the study. RJM reports receiving grant funding from Verily Life Sciences, Sergey Brin Family Foundation, and Texas Health Resources Clinical Scholar, outside of the submitted work; RJM also served on the advisory committee of Infectious Diseases Society of America - Digital Strategy Advisory Group, during the conduct of the study. BKN reports receiving grant funding from NIH, Veterans Affairs -Health Services Research and Development Service, the American Heart Association (AHA), Janssen, and Apple, outside of the submitted work; BKN also received compensation as editor in chief of Circulation: Cardiovascular Quality and Outcomes, a journal of AHA, during the conduct of the study; BKN is also a co-inventor on US Utility Patent No US15/356 012 (US20170148158A1) entitled “Automated Analysis of Vasculature in Coronary Angiograms,” that uses software technology with signal processing and machine learning to automate the reading of coronary angiograms, held by the University of Michigan; the patent is licensed to AngioInsight, in which BKN holds ownership shares and receives consultancy fees. EÖ reports having a patent pending for the University of Michigan for an artificial intelligence based approach for the dynamic prediction of health states for patients with occupational injuries. SNS reports serving on the editorial board for the Journal of the American Medical Informatics Association, and on the student editorial board for Applied Informatics Journal, during the conduct of the study. KS reports receiving grant funding from Blue Cross Blue Shield of Michigan, and Teva Pharmaceuticals, outside of the submitted work; KS also serves on a scientific advisory board for Flatiron Health, where he receives consulting fees and honorariums for invited lectures, during the conduct of the study. MWS reports serving on the planning committee for the Machine Learning for Healthcare Conference (MLHC), a non-profit organization that hosts a yearly academic meeting. JW reports receiving grant funding from Cisco Systems, D Dan and Betty Kahn Foundation, and Alfred P Sloan Foundation, during the conduct of the study outside of the submitted work; JW also served on the international advisory board for Lancet Digital Health, and on the advisory board for MLHC, during the conduct of the study. No other disclosures were reported that could appear to have influenced the submitted work. SD, JG, FK, BYL, XL, DSM, ESS, ST, TSV, and LRW all declare: no additional support from any organization for the submitted work; no additional financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Predicting covid-19 outcomes.BMJ. 2022 Feb 17;376:o354. doi: 10.1136/bmj.o354. BMJ. 2022. PMID: 35177413 No abstract available.
Similar articles
-
Machine Learning to Predict Mortality and Critical Events in a Cohort of Patients With COVID-19 in New York City: Model Development and Validation.J Med Internet Res. 2020 Nov 6;22(11):e24018. doi: 10.2196/24018. J Med Internet Res. 2020. PMID: 33027032 Free PMC article.
-
Learning From Past Respiratory Infections to Predict COVID-19 Outcomes: Retrospective Study.J Med Internet Res. 2021 Feb 22;23(2):e23026. doi: 10.2196/23026. J Med Internet Res. 2021. PMID: 33534724 Free PMC article.
-
Augmenting existing deterioration indices with chest radiographs to predict clinical deterioration.PLoS One. 2022 Feb 15;17(2):e0263922. doi: 10.1371/journal.pone.0263922. eCollection 2022. PLoS One. 2022. PMID: 35167608 Free PMC article.
-
External validation of six COVID-19 prognostic models for predicting mortality risk in older populations in a hospital, primary care, and nursing home setting.J Clin Epidemiol. 2024 Apr;168:111270. doi: 10.1016/j.jclinepi.2024.111270. Epub 2024 Feb 2. J Clin Epidemiol. 2024. PMID: 38311188
-
External validation of AI-based scoring systems in the ICU: a systematic review and meta-analysis.BMC Med Inform Decis Mak. 2025 Jan 6;25(1):5. doi: 10.1186/s12911-024-02830-7. BMC Med Inform Decis Mak. 2025. PMID: 39762808 Free PMC article.
Cited by
-
Defining medical liability when artificial intelligence is applied on diagnostic algorithms: a systematic review.Front Med (Lausanne). 2023 Nov 27;10:1305756. doi: 10.3389/fmed.2023.1305756. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38089864 Free PMC article. Review.
-
Predicting Severe Respiratory Failure in Patients with COVID-19: A Machine Learning Approach.J Clin Med. 2024 Dec 4;13(23):7386. doi: 10.3390/jcm13237386. J Clin Med. 2024. PMID: 39685844 Free PMC article.
-
From Biased Selective Labels to Pseudo-Labels: An Expectation-Maximization Framework for Learning from Biased Decisions.Proc Mach Learn Res. 2024 Jul;235:6286-6324. Proc Mach Learn Res. 2024. PMID: 40574796 Free PMC article.
-
Transforming Cardiovascular Care With Artificial Intelligence: From Discovery to Practice: JACC State-of-the-Art Review.J Am Coll Cardiol. 2024 Jul 2;84(1):97-114. doi: 10.1016/j.jacc.2024.05.003. J Am Coll Cardiol. 2024. PMID: 38925729 Free PMC article. Review.
-
Methodological challenges using routine clinical care data for real-world evidence: a rapid review utilizing a systematic literature search and focus group discussion.BMC Med Res Methodol. 2025 Jan 14;25(1):8. doi: 10.1186/s12874-024-02440-x. BMC Med Res Methodol. 2025. PMID: 39810151 Free PMC article.
