Assessment of the Cow's Milk-related Symptom Score (CoMiSS) as a diagnostic tool for cow's milk protein allergy: a prospective, multicentre study in China (MOSAIC study)
- PMID: 35177461
- PMCID: PMC8860045
- DOI: 10.1136/bmjopen-2021-056641
Assessment of the Cow's Milk-related Symptom Score (CoMiSS) as a diagnostic tool for cow's milk protein allergy: a prospective, multicentre study in China (MOSAIC study)
Abstract
Objectives: The MOSAIC study aimed to evaluate if the Cow's Milk-related Symptom Score (CoMiSS) can be used as a stand-alone diagnostic tool for cow's milk protein allergy (CMPA).
Design: Single-blinded, prospective, multicentre diagnostic accuracy study.
Setting: 10 paediatric centres in China.
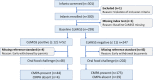
Participants: 300 non-breastfed infants (median age 16.1 weeks) with suspected CMPA.
Interventions: After performing the baseline CoMiSS, infants commenced a cow's milk protein elimination diet with amino acid-based formula for 14 days. CoMiSS was repeated at the end of the elimination trial. Infants then underwent an open oral food challenge (OFC) with cow's milk-based formula (CMF) in hospital. Infants who did not react during the OFC also completed a 14-day home challenge with CMF. A diagnosis of CMPA was made if acute or delayed reactions were reported.
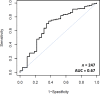
Primary outcome measures: A logistic regression model for CoMiSS to predict CMPA was fitted and a receiver-operator characteristic (ROC) curve generated. An area under the curve (AUC) of ≥0.75 was deemed adequate to validate CoMiSS as a diagnostic tool (target sensitivity 80%-90% and specificity 60%-70%).
Results: Of 254 infants who commenced the OFC, 250 completed both challenges, and a diagnosis of CMPA made in 217 (85.4%). The median baseline CoMiSS in this group fell from 8 (IQR 5-10) to 5 (IQR 3-7) at visit 2 (p<0.000000001), with a median change of -3 (IQR -6 to -1). A baseline CoMiSS of ≥12 had a low sensitivity (20.3%), but high specificity (87.9%) and high positive predictive value (91.7%) for CMPA. The ROC analysis with an AUC of 0.67 fell short of the predefined primary endpoint.
Conclusions: The present study did not support the use of CoMiSS as a stand-alone diagnostic tool for CMPA. Nevertheless, CoMiSS remains a clinically useful awareness tool to help identify infants with cow's milk-related symptoms.
Trial registration number: NCT03004729; Pre-results.
Keywords: allergy; community child health; paediatric dermatology; paediatric gastroenterology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: YV has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Abbott Nutrition, Biogaia, Biocodex, Danone, Hero, Hipp, Johnson & Johnson, Mead Johnson, Merck, Menarini, Nestlé Nutrition Institute, Nutricia, Orafti, Pfizer, Phacobel, Sari Husada, Shire (Movetis), Sucampo, Takeda, United Pharmaceuticals, Wyeth and Yakult. CD has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Abbott Nutrition, Danone, Novalac, Nestlé Nutrition Institute, Nutricia, Sodilac, United Pharmaceuticals and Wyeth. PE has received lecture honoraria from Danone and Sodilac, and has received research grants from Nestlé. CR-K has participated as consultant and/or speaker for Mead Johnson International, Hero Institute, Alter-Nutriben, Danone and Nestlé. HS has participated as a clinical investigator and/or speaker for Ausnutria, Arla, Biogaia, Biocodex, Danone, Dicofarm, Else Nutrition, HiPP, Nestlé, Nestlé Nutrition Institute, Nutricia, Mead Johnson, Myor and Merck. KB has received lecture honoraria from Nestlé. SS has participated as a clinical investigator/, and/or advisory board member, and/or consultant, and/or speaker for Danone, Dicofarm, DVA, Nestlé and United Pharmaceuticals. RS has participated as clinical investigator, and/or advisory board member, and/or consultant and/or speaker for Abbott, Else, Nestlé Nutrition Institute, NGS and Nutricia. AJ and RGH are full-time employees of Nestlé Health Science. The other authors have disclosed no conflict of interest relevant to this article.
Figures

Similar articles
-
Cow's Milk-related Symptom Score for cow's milk allergy assessment: a meta-analysis for test accuracy.Pediatr Res. 2023 Mar;93(4):772-779. doi: 10.1038/s41390-022-02334-y. Epub 2022 Oct 17. Pediatr Res. 2023. PMID: 36253506
-
Evaluation of Cow's Milk Related Symptom Score [CoMiSS] accuracy in cow's milk allergy diagnosis.Pediatr Res. 2023 Sep;94(3):987-995. doi: 10.1038/s41390-023-02539-9. Epub 2023 Mar 4. Pediatr Res. 2023. PMID: 36871030 Free PMC article.
-
Protocol for the validation of sensitivity and specificity of the Cow's Milk-related Symptom Score (CoMiSS) against open food challenge in a single-blinded, prospective, multicentre trial in infants.BMJ Open. 2018 May 17;8(5):e019968. doi: 10.1136/bmjopen-2017-019968. BMJ Open. 2018. PMID: 29773698 Free PMC article.
-
Assessment of Cow's milk-related symptom scores in early identification of cow's milk protein allergy in Chinese infants.BMC Pediatr. 2019 Jun 10;19(1):191. doi: 10.1186/s12887-019-1563-y. BMC Pediatr. 2019. PMID: 31179927 Free PMC article.
-
The Cow's Milk-Related Symptom Score (CoMiSS™): A Useful Awareness Tool.Nutrients. 2022 May 14;14(10):2059. doi: 10.3390/nu14102059. Nutrients. 2022. PMID: 35631201 Free PMC article. Review.
Cited by
-
Cow's Milk-related Symptom Score for cow's milk allergy assessment: a meta-analysis for test accuracy.Pediatr Res. 2023 Mar;93(4):772-779. doi: 10.1038/s41390-022-02334-y. Epub 2022 Oct 17. Pediatr Res. 2023. PMID: 36253506
-
The Naples pediatric food allergy (NAPFA) score: A multivariable model for the prediction of food allergy in children.Pediatr Allergy Immunol. 2025 Apr;36(4):e70071. doi: 10.1111/pai.70071. Pediatr Allergy Immunol. 2025. PMID: 40162580 Free PMC article.
-
Evaluation of Cow's Milk Related Symptom Score [CoMiSS] accuracy in cow's milk allergy diagnosis.Pediatr Res. 2023 Sep;94(3):987-995. doi: 10.1038/s41390-023-02539-9. Epub 2023 Mar 4. Pediatr Res. 2023. PMID: 36871030 Free PMC article.
-
Effects of an Extensively Hydrolyzed Formula Supplemented with Two Human Milk Oligosaccharides on Growth, Tolerability, Safety and Infection Risk in Infants with Cow's Milk Protein Allergy: A Randomized, Multi-Center Trial.Nutrients. 2022 Jan 26;14(3):530. doi: 10.3390/nu14030530. Nutrients. 2022. PMID: 35276889 Free PMC article. Clinical Trial.
-
Managing Cow's Milk Protein Allergy with an Extensively Hydrolyzed Formula: Results from a Prospective, Non-Interventional Study in France (EVA Study).Nutrients. 2022 Mar 12;14(6):1203. doi: 10.3390/nu14061203. Nutrients. 2022. PMID: 35334859 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
