The m6A reader YTHDC2 is essential for escape from KSHV SOX-induced RNA decay
- PMID: 35177478
- PMCID: PMC8872733
- DOI: 10.1073/pnas.2116662119
The m6A reader YTHDC2 is essential for escape from KSHV SOX-induced RNA decay
Abstract
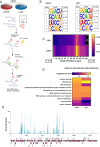
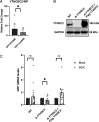
The role of N6-methyladenosine (m6A) modifications has increasingly been associated with a diverse set of roles in modulating viruses and influencing the outcomes of viral infection. Here, we report that the landscape of m6A deposition is drastically shifted during Kaposi's sarcoma-associated herpesvirus (KSHV) lytic infection for both viral and host transcripts. In line with previous reports, we also saw an overall decrease in host methylation in favor of viral messenger RNA (mRNA), along with 5' hypomethylation and 3' hypermethylation. During KSHV lytic infection, a major shift in overall mRNA abundance is driven by the viral endoribonuclease SOX, which induces the decay of greater than 70% of transcripts. Here, we reveal that interlukin-6 (IL-6) mRNA, a well-characterized, SOX-resistant transcript, is m6A modified during lytic infection. Furthermore, we show that this modification falls within the IL-6 SOX resistance element, an RNA element in the IL-6 3' untranslated region (UTR) that was previously shown to be sufficient for protection from SOX cleavage. We show that the presence of this m6A modification is essential to confer SOX resistance to the IL-6 mRNA. We next show that this modification recruits the m6A reader YTHDC2 and found that YTHDC2 is necessary for the escape of the IL-6 transcript. These results shed light on how the host cell has evolved to use RNA modifications to circumvent viral manipulation of RNA fate during KSHV infection.
Keywords: IL-6; RNA decay; herpesvirus; m6A; m6A readers.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
The role of N6-methyladenosine (m6A) mRNA modifications in herpesvirus infections.J Virol. 2025 Feb 25;99(2):e0172324. doi: 10.1128/jvi.01723-24. Epub 2025 Jan 27. J Virol. 2025. PMID: 39868828 Free PMC article. Review.
-
C19ORF66 Broadly Escapes Virus-Induced Endonuclease Cleavage and Restricts Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2019 May 29;93(12):e00373-19. doi: 10.1128/JVI.00373-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30944177 Free PMC article.
-
Viral and cellular N6-methyladenosine and N6,2'-O-dimethyladenosine epitranscriptomes in the KSHV life cycle.Nat Microbiol. 2018 Jan;3(1):108-120. doi: 10.1038/s41564-017-0056-8. Epub 2017 Nov 6. Nat Microbiol. 2018. PMID: 29109479 Free PMC article.
-
A ribonucleoprotein complex protects the interleukin-6 mRNA from degradation by distinct herpesviral endonucleases.PLoS Pathog. 2015 May 12;11(5):e1004899. doi: 10.1371/journal.ppat.1004899. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25965334 Free PMC article.
-
The RNA Epitranscriptome of DNA Viruses.J Virol. 2018 Oct 29;92(22):e00696-18. doi: 10.1128/JVI.00696-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30185592 Free PMC article. Review.
Cited by
-
The role of N6-methyladenosine (m6A) mRNA modifications in herpesvirus infections.J Virol. 2025 Feb 25;99(2):e0172324. doi: 10.1128/jvi.01723-24. Epub 2025 Jan 27. J Virol. 2025. PMID: 39868828 Free PMC article. Review.
-
Interferon induced circRNAs escape herpesvirus host shutoff and suppress lytic infection.EMBO Rep. 2024 Mar;25(3):1541-1569. doi: 10.1038/s44319-023-00051-z. Epub 2024 Jan 23. EMBO Rep. 2024. PMID: 38263330 Free PMC article.
-
The biological function of the N6-Methyladenosine reader YTHDC2 and its role in diseases.J Transl Med. 2024 May 24;22(1):490. doi: 10.1186/s12967-024-05293-6. J Transl Med. 2024. PMID: 38790013 Free PMC article. Review.
-
Recent insights into N6-methyladenosine during viral infection.Curr Opin Genet Dev. 2024 Aug;87:102213. doi: 10.1016/j.gde.2024.102213. Epub 2024 Jun 19. Curr Opin Genet Dev. 2024. PMID: 38901100 Free PMC article. Review.
-
RNA m6A modification in liver biology and its implication in hepatic diseases and carcinogenesis.Am J Physiol Cell Physiol. 2022 Oct 1;323(4):C1190-C1205. doi: 10.1152/ajpcell.00214.2022. Epub 2022 Aug 29. Am J Physiol Cell Physiol. 2022. PMID: 36036444 Free PMC article. Review.
References
-
- Glaunsinger B., Ganem D., Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell 13, 713–723 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

