Performance Evaluation of the Aptima Assays in Comparison with the cobas 6800 Assays for the Detection of HIV-1, HBV, and HCV in Clinical Samples
- PMID: 35177565
- PMCID: PMC8859551
- DOI: 10.3343/alm.2022.42.4.447
Performance Evaluation of the Aptima Assays in Comparison with the cobas 6800 Assays for the Detection of HIV-1, HBV, and HCV in Clinical Samples
Abstract
Background: Accurate and consistent viral load (VL) quantitation of HIV type 1 (HIV-1), hepatitis B virus (HBV), and hepatitis C virus (HCV) is important for diagnosis and clinical monitoring. Assay results have to be concordant and compatible across laboratories. We evaluated the performance of three Aptima assays (Hologic, San Diego, CA, USA) and compared their VL values with corresponding cobas 6800 assay (Roche Diagnostics, Mannheim, Germany) results, using 840 clinical samples.
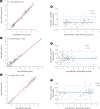
Methods: The correlation between VL results obtained using the two assays was evaluated in terms of analytical sensitivity, precision/reproducibility, linearity, and cross-reactivity. Agreement rates were determined using kappa statistics. The overall agreement of VL values was examined using Passing-Bablok regression analysis.
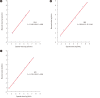
Results: All CVs were within 5%; the assays had good precision for detecting all three viruses. The linearity of quantitation assessed using three AccuSpan linearity panels (Seracare, Milford, MA, USA), was excellent for the Aptima assays. For HIV-1 and HCV, the results of both assays showed excellent agreement (κ=0.89 and 0.90, respectively) while for HBV, the results showed good agreement (κ=0.69). For analytical sensitivity, the VLs required for a 100% detection rate of HIV-1, HBV, and HCV were 20 copies/mL, 7.5 IU/mL, and 5.0 IU/mL, respectively. The results for HIV-1, HBV, and HCV obtained using both assays correlated strongly (R2=0.97, 0.93, and 0.95, respectively).
Conclusions: The cobas 6800 and Aptima assays, with fully automated and high-throughput molecular platforms for HIV-1, HBV, and HCV VL measurements, show good analytical performance and a strong correlation between results. The study results suggest that the assays can be used interchangeably for long-term monitoring of chronic infections.
Keywords: Agreement; Analytical sensitivity; Aptima assay; HIV; Hepatitis B virus; Hepatitis C virus; Performance; Viral load; cobas 6800 assay.
Conflict of interest statement
No potential conflicts of interest relevant to this study are reported.
Figures
Similar articles
-
Clinical performance evaluation of the Aptima viral assays for the quantitation of HIV-1, HCV, and HBV in plasma samples.Diagn Microbiol Infect Dis. 2023 Jul;106(3):115951. doi: 10.1016/j.diagmicrobio.2023.115951. Epub 2023 Apr 5. Diagn Microbiol Infect Dis. 2023. PMID: 37127022
-
Analytical characteristics and comparative evaluation of Aptima HCV quant Dx assay with the Abbott RealTime HCV assay and Roche COBAS AmpliPrep/COBAS TaqMan HCV quantitative test v2.0.Virol J. 2017 Apr 4;14(1):66. doi: 10.1186/s12985-017-0727-3. Virol J. 2017. PMID: 28372576 Free PMC article.
-
Analytical performance of four molecular platforms used for HIV-1, HBV and HCV viral load determinations.Expert Rev Mol Diagn. 2019 Oct;19(10):941-949. doi: 10.1080/14737159.2019.1624162. Epub 2019 Jun 19. Expert Rev Mol Diagn. 2019. PMID: 31159598
-
Performance of the New Aptima HCV Quant Dx Assay in Comparison to the Cobas TaqMan HCV2 Test for Use with the High Pure System in Detection and Quantification of Hepatitis C Virus RNA in Plasma or Serum.J Clin Microbiol. 2016 Apr;54(4):1101-7. doi: 10.1128/JCM.03236-15. Epub 2016 Feb 10. J Clin Microbiol. 2016. PMID: 26865682 Free PMC article.
-
Mini review: current molecular methods for the detection and quantification of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus type 1.Int J Infect Dis. 2014 Aug;25:145-9. doi: 10.1016/j.ijid.2014.04.007. Epub 2014 Jun 10. Int J Infect Dis. 2014. PMID: 24927665 Review.
Cited by
-
Performance Evaluation of the Roche Cobas 5800 HBV and HCV Tests: Comparison of the 200 and 500 μL Protocols.Ann Lab Med. 2024 May 1;44(3):253-261. doi: 10.3343/alm.2023.0306. Epub 2023 Dec 15. Ann Lab Med. 2024. PMID: 38098301 Free PMC article.
-
Validation of a Real-Time PCR Assay for Fully Automated Detection of Bacillus cereus in Donor Human Milk.Microorganisms. 2025 Jul 11;13(7):1640. doi: 10.3390/microorganisms13071640. Microorganisms. 2025. PMID: 40732149 Free PMC article.
-
Multiplex Detection of RNA Viruses Based on Ligation Reaction and Universal PCR Amplification.Curr Microbiol. 2024 Jan 23;81(3):75. doi: 10.1007/s00284-023-03582-9. Curr Microbiol. 2024. PMID: 38261072
-
The Perils of Overly Sensitive Viral Load Testing for Persons With Human Immunodeficiency Virus.Open Forum Infect Dis. 2023 Oct 3;10(10):ofad494. doi: 10.1093/ofid/ofad494. eCollection 2023 Oct. Open Forum Infect Dis. 2023. PMID: 37849507 Free PMC article.
-
Detection of anti-HCV antibodies in the clinical classification and epidemiological surveillance of HCV infection.Mol Biol Rep. 2025 Jul 17;52(1):730. doi: 10.1007/s11033-025-10827-2. Mol Biol Rep. 2025. PMID: 40676404 Review.
References
-
- World Health Organization, author. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed [Internet] World Health Organization; Geneva: 2016. [cited 2021 Dec 12]. Available from: https://www.who.int/publications/i/item/9789241549684 . - PubMed
-
- European Association for the Study of the Liver, author. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. - PubMed
-
- European Association for the Study of the Liver, author. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. - PubMed
-
- European Association for the Study of the Liver, author. Electronic address: easloffice@easloffice.eu; Clinical Practice Guidelines Panel: Chair:, EASL Governing Board representative:, Panel members. EASL recommendations on treatment of hepatitis C: final update of the series☆. J Hepatol. 2020;73:1170–218. doi: 10.1016/j.jhep.2020.08.018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

