Presynaptic NMDA Receptors Influence Ca2+ Dynamics by Interacting with Voltage-Dependent Calcium Channels during the Induction of Long-Term Depression
- PMID: 35178084
- PMCID: PMC8844386
- DOI: 10.1155/2022/2900875
Presynaptic NMDA Receptors Influence Ca2+ Dynamics by Interacting with Voltage-Dependent Calcium Channels during the Induction of Long-Term Depression
Abstract
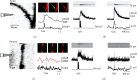
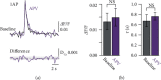
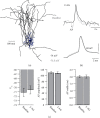
Spike-timing-dependent long-term depression (t-LTD) of glutamatergic layer (L)4-L2/3 synapses in developing neocortex requires activation of astrocytes by endocannabinoids (eCBs), which release glutamate onto presynaptic NMDA receptors (preNMDARs). The exact function of preNMDARs in this context is still elusive and strongly debated. To elucidate their function, we show that bath application of the eCB 2-arachidonylglycerol (2-AG) induces a preNMDAR-dependent form of chemically induced LTD (eCB-LTD) in L2/3 pyramidal neurons in the juvenile somatosensory cortex of rats. Presynaptic Ca2+ imaging from L4 spiny stellate axons revealed that action potential (AP) evoked Ca2+ transients show a preNMDAR-dependent broadening during eCB-LTD induction. However, blockade of voltage-dependent Ca2+ channels (VDCCs) did not uncover direct preNMDAR-mediated Ca2+ transients in the axon. This suggests that astrocyte-mediated glutamate release onto preNMDARs does not result in a direct Ca2+ influx, but that it instead leads to an indirect interaction with presynaptic VDCCs, boosting axonal Ca2+ influx. These results reveal one of the main remaining missing pieces in the signaling cascade of t-LTD at developing cortical synapses.
Copyright © 2022 Florian B. Neubauer et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Bouvier G., Bidoret C., Casado M., Paoletti P. Presynaptic NMDA receptors: roles and rules. Neuroscience . 2015;311:322–340. - PubMed
-
- Sjostrom P. J., Turrigiano G. G., Nelson S. B. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron . 2003;39(4):641–664. - PubMed
-
- Rodriguez-Moreno A., Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nature Neuroscience . 2008;11(7):744–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

