Hammerhead flatworms (Platyhelminthes, Geoplanidae, Bipaliinae): mitochondrial genomes and description of two new species from France, Italy, and Mayotte
- PMID: 35178290
- PMCID: PMC8815365
- DOI: 10.7717/peerj.12725
Hammerhead flatworms (Platyhelminthes, Geoplanidae, Bipaliinae): mitochondrial genomes and description of two new species from France, Italy, and Mayotte
Abstract
Background: New records of alien land planarians are regularly reported worldwide, and some correspond to undescribed species of unknown geographic origin. The description of new species of land planarians (Geoplanidae) should classically be based on both external morphology and histology of anatomical structures, especially the copulatory organs, ideally with the addition of molecular data.
Methods: Here, we describe the morphology and reproductive anatomy of a species previously reported as Diversibipalium "black", and the morphology of a species previously reported as Diversibipalium "blue". Based on next generation sequencing, we obtained the complete mitogenome of five species of Bipaliinae, including these two species.
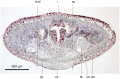
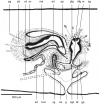
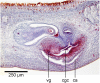
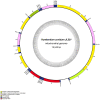
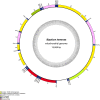
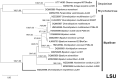
Results: The new species Humbertium covidum n. sp. (syn: Diversibipalium "black" of Justine et al., 2018) is formally described on the basis of morphology, histology and mitogenome, and is assigned to Humbertium on the basis of its reproductive anatomy. The type-locality is Casier, Italy, and other localities are in the Department of Pyrénées-Atlantiques, France; some published or unpublished records suggest that this species might also be present in Russia, China, and Japan. The mitogenomic polymorphism of two geographically distinct specimens (Italy vs France) is described; the cox1 gene displayed 2.25% difference. The new species Diversibipalium mayottensis n. sp. (syn: Diversibipalium "blue" of Justine et al., 2018) is formally described on the basis of external morphology and complete mitogenome and is assigned to Diversibipalium on the basis of an absence of information on its reproductive anatomy. The type- and only known locality is the island of Mayotte in the Mozambique Channel off Africa. Phylogenies of bipaliine geoplanids were constructed on the basis of SSU, LSU, mitochondrial proteins and concatenated sequences of cox1, SSU and LSU. In all four phylogenies, D. mayottensis was the sister-group to all the other bipaliines. With the exception of D. multilineatum which could not be circularised, the complete mitogenomes of B. kewense, B. vagum, B. adventitium, H. covidum and D. mayottensis were colinear. The 16S gene in all bipaliine species was problematic because usual tools were unable to locate its exact position.
Conclusion: Next generation sequencing, which can provide complete mitochondrial genomes as well as traditionally used genes such as SSU, LSU and cox1, is a powerful tool for delineating and describing species of Bipaliinae when the reproductive structure cannot be studied, which is sometimes the case of asexually reproducing invasive species. The unexpected position of the new species D. mayottensis as sister-group to all other Bipaliinae in all phylogenetic analyses suggests that the species could belong to a new genus, yet to be described.
Keywords: Alien invasive species; Barcoding; Citizen science; France; Italy; Land planarians; Mayotte; Mitogenome; Platyhelminthes; Taxonomy.
© 2022 Justine et al.
Conflict of interest statement
Jean-Lou Justine is an Academic Editor of PeerJ.
Figures


































References
-
- Andújar C, Creedy TJ, Arribas P, López H, Salces-Castellano A, Pérez-Delgado AJ, Vogler AP, Emerson BC. Validated removal of nuclear pseudogenes and sequencing artefacts from mitochondrial metabarcode data. Molecular Ecology Resources. 2021;21(6):1772–1787. doi: 10.1111/1755-0998.13337. - DOI - PubMed
-
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

