IGF Signaling in Intervertebral Disc Health and Disease
- PMID: 35178405
- PMCID: PMC8843937
- DOI: 10.3389/fcell.2021.817099
IGF Signaling in Intervertebral Disc Health and Disease
Abstract
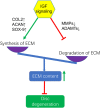
Low back pain (LBP) is a common musculoskeletal symptom, which brings a lot of pain and economic loss to patients. One of the most common causes of LBP is intervertebral disc degeneration (IVDD). However, pathogenesis is still debated, and therapeutic options are limited. Insulin-like growth factor (IGF) signaling pathways play an important role in regulating different cell processes, including proliferation, differentiation, migration, or cell death, which are critical to the homeostasis of tissues and organs. The IGF signaling is crucial in the occurrence and progression of IVDD. The activation of IGF signaling retards IVDD by increasing cell proliferation, promoting extracellular matrix (ECM) synthesis, inhibiting ECM decomposition, and preventing apoptosis and senescence of disc cells. However, abnormal activation of IGF signaling may promote the process of IVDD. IGF signaling is currently considered to have a promising treatment prospect for IVDD. An in-depth understanding of the role of IGF signaling in IVDD may help find a novel approach for IVDD treatment.
Keywords: degeneration; insulin-like growth factor; intervertebral disc; low back pain; nucleus pulposus.
Copyright © 2022 Lin, Tian, Peng, Wu, Xiao, Qing and Shao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bergknut N., Smolders L. A., Grinwis G. C. M., Hagman R., Lagerstedt A.-S., Hazewinkel H. A. W., et al. (2013). Intervertebral Disc Degeneration in the Dog. Part 1: Anatomy and Physiology of the Intervertebral Disc and Characteristics of Intervertebral Disc Degeneration. Vet. J. 195, 282–291. 10.1016/j.tvjl.2012.10.024 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

