Immigrant IBD Patients in Spain Are Younger, Have More Extraintestinal Manifestations and Use More Biologics Than Native Patients
- PMID: 35178413
- PMCID: PMC8844561
- DOI: 10.3389/fmed.2022.823900
Immigrant IBD Patients in Spain Are Younger, Have More Extraintestinal Manifestations and Use More Biologics Than Native Patients
Abstract
Background: Previous studies comparing immigrant ethnic groups and native patients with IBD have yielded clinical and phenotypic differences. To date, no study has focused on the immigrant IBD population in Spain.
Methods: Prospective, observational, multicenter study comparing cohorts of IBD patients from ENEIDA-registry who were born outside Spain with a cohort of native patients.
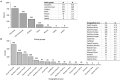
Results: We included 13,524 patients (1,864 immigrant and 11,660 native). The immigrants were younger (45 ± 12 vs. 54 ± 16 years, p < 0.001), had been diagnosed younger (31 ± 12 vs. 36 ± 15 years, p < 0.001), and had a shorter disease duration (14 ± 7 vs. 18 ± 8 years, p < 0.001) than native patients. Family history of IBD (9 vs. 14%, p < 0.001) and smoking (30 vs. 40%, p < 0.001) were more frequent among native patients. The most prevalent ethnic groups among immigrants were Caucasian (41.5%), followed by Latin American (30.8%), Arab (18.3%), and Asian (6.7%). Extraintestinal manifestations, mainly musculoskeletal affections, were more frequent in immigrants (19 vs. 11%, p < 0.001). Use of biologics, mainly anti-TNF, was greater in immigrants (36 vs. 29%, p < 0.001). The risk of having extraintestinal manifestations [OR: 2.23 (1.92-2.58, p < 0.001)] and using biologics [OR: 1.13 (1.0-1.26, p = 0.042)] was independently associated with immigrant status in the multivariate analyses.
Conclusions: Compared with native-born patients, first-generation-immigrant IBD patients in Spain were younger at disease onset and showed an increased risk of having extraintestinal manifestations and using biologics. Our study suggests a featured phenotype of immigrant IBD patients in Spain, and constitutes a new landmark in the epidemiological characterization of immigrant IBD populations in Southern Europe.
Keywords: Crohn's disease; biologics; immigrant; inflammatory bowel disease; phenotype; ulcerative colitis.
Copyright © 2022 Gutiérrez, Zapater, Ricart, González-Vivó, Gordillo, Olivares, Vera, Mañosa, Gisbert, Aguas, Sánchez-Rodríguez, Bosca-Watts, Laredo, Camps, Marín-Jiménez, Zabana, Martín-Arranz, Muñoz, Navarro, Sierra, Madero, Vela, Pérez-Calle, Sainz, Calvet, Arias, Morales, Bermejo, Fernández-Salazar, Van Domselaar, De Castro, Rodríguez, Muñoz-Villafranca, Lorente, Rivero, Iglesias, Herreros, Busquets, Riera, Martínez-Montiel, Roldón, Roncero, Hinojosa, Sierra, Barrio, De Francisco, Huguet, Merino, Carpio, Ginard, Muñoz, Piqueras, Almela, Argüelles-Arias, Alcaín, Bujanda, Manceñido, Lucendo, Varela, Rodríguez-Lago, Ramos, Sempere, Sesé, Barreiro-de Acosta, Domènech and Francés.
Conflict of interest statement
AG has served as speaker, consultant and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Tillotts Pharma, Chiesi and Otsuka Pharmaceutical. ER has provided scientific advice/participated in medical meetings/received research funding from/received payment for presentations and advice from: MSD, Schering-Plow, Ferring, Abbvie, Takeda, Janssen, Fresenius Kabi, Pfizer. IV has served as a speaker, or has received research or education funding from Abbvie, MSD, Pfizer, Takeda, Janssen, Tillotts Pharma, Ferring and Shire Pharmaceuticals. MM has served as speaker or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Biogen, Tillotts Pharma, Chiesi and Adacyte. JPG has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene, Gilead/Galapagos, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, and Vifor Pharma. YZ has received support for conference attendance, speaker fees, research support and consulting fees from Abbvie, Adacyte, Almirall, Amgen, Dr. Falk, FAES Pharma, Ferring, Janssen, MSD, Otsuka, Pfizer, Shire, Takeda and Tillots. XC has received grants for research from Abbvie, MSD, Vifor fees for advisory boards form Abbvie, MSD, Takeda, Pfizer, Janssen and VIFOR and has given lectures for Abbvie, MSD, Janssen, Pfizer, Takeda, Shire and Allergan. FB has served as a speaker, a consultant and advisory member for or has received research funding from MSD, Abbvie, Takeda, Janssen, Pfizer, Biogen, Amgen, Ferring, Faes Farma, Tillotts Pharma, Falk Pharma, Chiesi, Gebro Pharma, Vifor Pharma. MM-M has served as speaker, consultant and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Shire Pharmaceuticals and Otsuka Pharmaceutical. MR has served as speaker, consultant or advisory member for MSD, Abbvie, Pfizer, Takeda, Janssen, Ferring and Chiesi. MP has served as speaker or has received research funding from Abbvie, Takeda and Janssen. IR-L has received financial support for traveling and educational activities from or has served as an advisory board member for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Shire Pharmaceuticals, Roche, Celltrion, Faes Farma, Ferring, Dr. Falk Pharma, Otsuka Pharmaceutical and Adacyte. Financial support for research from Tillotts Pharma. RL has served as a speaker, or has received research or education funding from MSD, Abbvie, Pfizer, Takeda, Janssen and Dr. Falk. LA has served as speaker, or has received research or education funding from MSD, Abbvie, Kern Pharma, Ferring, FaesFarma, Shire Pharmaceuticals, Pfizer, Takeda, Janssen, Tillotts Pharma, and Otsuka Pharmaceutical. FA-A has served as speaker, consultant and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Tillotts Pharma, Chiesi and Dr. Falk. LR has served as a speaker, or has received education funding from MSD, Abbvie, Adacyte, Takeda, Pfizer, Janssen and Ferring. MB-d has served as a speaker, consultant and advisory member for or has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gillead, Celgene, Pfizer, Sandoz, Biogen, Fresenius, Ferring, Faes Farma, Dr. Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma. ED has served as a speaker, or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Gilead, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, Tillots, Thermofisher. RF has served as a speaker, or has received research or education funding or advisory fees from AbbVie, Janssen, Takeda, Adacyte Therapeutics, Sanofi, GlaxoSmithKline, Almirall. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures