Complications of Cosmetic Botulinum Toxin A Injections to the Upper Face: A Systematic Review and Meta-Analysis
- PMID: 35178552
- PMCID: PMC9005453
- DOI: 10.1093/asj/sjac036
Complications of Cosmetic Botulinum Toxin A Injections to the Upper Face: A Systematic Review and Meta-Analysis
Abstract
Background: Botulinum toxin A (BoNT-A) injections are a popular non-surgical procedure for facial rejuvenation. Its increase in popularity and utilization is met with limited regulations, potentially posing a significant risk to patient safety and public health.
Objectives: The authors sought to assess the safety profile of cosmetic glabellar and forehead BoNT-A injections and evaluate BoNT-A type on complication rate.
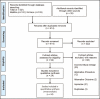
Methods: A systematic search of MEDLINE and EMBASE was performed for studies reporting complications after cosmetic BoNT-A in the glabellar or in the forehead region in the glabellar or in the forehead region. A random effects meta-analysis was carried out to assess complication rate. Where there were sufficient randomized-controlled trials, a network meta-analysis was performed.
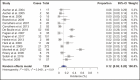
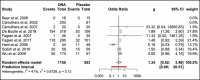
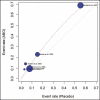
Results: Of 556 identified articles, 24 were included in the final quantitative analysis, with 4268 BoNT-A injection sessions and 1234 placebos. Frequently observed treatment-related complications in the BoNT-A intervention group included headache, local skin reactions, and facial neuromuscular symptoms. The overall BoNT-A complication rate was 16%. The odds ratio of developing complications from abobotulinum toxin injections compared with placebo was 1.62 (1.15, 2.27; P > 0.05) and that from onabotulinum toxin injections compared with placebo was 1.34 (0.52, 3.48; P > 0.05). In 30% of the studies, the injectors were doctors, whereas the training status of the practitioner was not reported in the remaining 70%.
Conclusions: Cosmetic BoNT-A injections in the glabellar and forehead region appear to be safe, and most complications are mild and transient. Nevertheless, the literature demonstrates heterogeneous reporting of complications and a lack of consistency of the definition of treatment-related complications.
© 2022 The Aesthetic Society.
Figures






References
-
- Statista. Size of the anti-aging market worldwide 2018-2023. Accessed November 30, 2020. https://www.statista.com/statistics/509679/value-of-the-global-anti-agin....
-
- Statista. U.S. patient satisfaction: top nonsurgical cosmetic procedures 2019. Accessed November 30, 2020. https://www.statista.com/statistics/281434/satisfaction-among-patients-o....
-
- BOTOX 100 units powder for solution for injection—summary of product characteristics (SmPC)—(emc). Accessed November 10, 2020. https://www.medicines.org.uk/emc/product/859/smpc#gref.
-
- Xeomin 200 units powder for solution for injection—summary of product characteristics (SmPC)—(emc). Accessed November 10, 2020. https://www.medicines.org.uk/emc/product/2162/smpc#gref.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

