Cell interactome in sarcopenia during aging
- PMID: 35178901
- PMCID: PMC8977965
- DOI: 10.1002/jcsm.12937
Cell interactome in sarcopenia during aging
Abstract
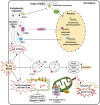
Background: The diversity between the muscle cellular interactome of dependent and independent elderly people is based on the interrelationships established between different cellular mechanisms, and alteration of this balance modulates cellular activity in muscle tissue with important functional implications.
Methods: Thirty patients (85 ± 8 years old, 23% female) scheduled to undergo hip fracture surgery participated in this study. During the surgical procedures, skeletal muscle tissue was obtained from the Vastus lateralis. Two groups of participants were studied based on their Barthel index: 15 functional-independent individuals (100-90) and 15 severely functional-dependent individuals (40-0). The expression of proteins from the most important cellular mechanisms was studied by western blot.
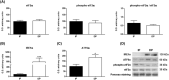
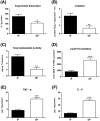
Results: Compared with independent elderly patients, dependent elderly showed an abrupt decrease in the capacity of protein synthesis; this decrease was only partially compensated for at the response to unfolded or misfolded proteins (UPR) level due to the increase in IRE1 (P < 0.001) and ATF6 (P < 0.05), which block autophagy, an essential mechanism for cell survival, by decreasing the expression of Beclin-1, LC3, and p62 (P < 0.001) and the antioxidant response. This lead to increased oxidative damage to lipids (P < 0.001) and that damage was directly associated with the mitochondrial impairment induced by the significant decreases in the I, III, IV, and V mitochondrial complexes (P < 0.01), which drastically reduced the energy capacity of the cell. The essential cellular mechanisms were generally impaired and the triggering of apoptosis was induced, as shown by the significantly elevated levels of most proapoptotic proteins (P < 0.05) and caspase-3/7 (P < 0.001) in dependents. The death of highly damaged cells is not detrimental to organs as long as the regenerative capacity remains unaltered, but in the dependent patients, this ability was also significantly altered, which was revealed by the reduction in the myogenic regulatory factors and satellite cell marker (P < 0.001), and the increase in myostatin (P < 0.01). Due to the severely disturbed cell interactome, the muscle contractile capacity showed significant damage.
Conclusions: Functionally dependent patients exhibited severe alterations in their cellular interactome at the muscle level. Cell apoptosis was caused by a decrease in successful protein synthesis, to which the cellular control systems did not respond adequately; autophagy was simultaneously blocked, the mitochondrion malfunctioned, and as the essential recovery mechanisms failed, these cells could not be replaced, resulting in the muscle being condemned to a loss of mass and functionality.
Keywords: Elderly; Mitochondria; Myogenic regulatory factors; Oxidative stress; Sarcopenia; Unfolded protein response.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short‐term disuse: implications for age‐related sarcopenia. Ageing Res Rev 2013;12:898–906. - PubMed
-
- Potes Y, de Luxán‐Delgado B, Rodriguez‐González S, Guimarães MRM, Solano JJ, Fernández‐Fernández M, et al. Overweight in elderly people induces impaired autophagy in skeletal muscle. Free Radic Biol Med 2017;110:31–41. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

