Myocardial Cytoskeletal Adaptations in Advanced Kidney Disease
- PMID: 35179046
- PMCID: PMC9075094
- DOI: 10.1161/JAHA.121.022991
Myocardial Cytoskeletal Adaptations in Advanced Kidney Disease
Erratum in
-
Correction to: Myocardial Cytoskeletal Adaptations in Advanced Kidney Disease.J Am Heart Assoc. 2023 Mar 21;12(6):e027645. doi: 10.1161/JAHA.121.027645. Epub 2023 Mar 9. J Am Heart Assoc. 2023. PMID: 36892070 Free PMC article. No abstract available.
Abstract
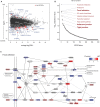
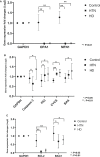
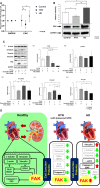
Background The myocardial cytoskeleton functions as the fundamental framework critical for organelle function, bioenergetics and myocardial remodeling. To date, impairment of the myocardial cytoskeleton occurring in the failing heart in patients with advanced chronic kidney disease has been largely undescribed. Methods and Results We conducted a 3-arm cross-sectional cohort study of explanted human heart tissues from patients who are dependent on hemodialysis (n=19), hypertension (n=10) with preserved renal function, and healthy controls (n=21). Left ventricular tissues were subjected to pathologic examination and next-generation RNA sequencing. Mechanistic and interference RNA studies utilizing in vitro human cardiac fibroblast models were performed. Left ventricular tissues from patients undergoing hemodialysis exhibited increased myocardial wall thickness and significantly greater fibrosis compared with hypertension patients (P<0.05) and control (P<0.01). Transcriptomic analysis revealed that the focal adhesion pathway was significantly enriched in hearts from patients undergoing hemodialysis. Hearts from patients undergoing hemodialysis exhibited dysregulated components of the focal adhesion pathway including reduced β-actin (P<0.01), β-tubulin (P<0.01), vimentin (P<0.05), and increased expression of vinculin (P<0.05) compared with controls. Cytoskeletal adaptations in hearts from the hemodialysis group were associated with impaired mitochondrial bioenergetics, including dysregulated mitochondrial dynamics and fusion, and loss of cell survival pathways. Mechanistic studies revealed that cytoskeletal changes can be driven by uremic and metabolic abnormalities of chronic kidney disease, in vitro. Furthermore, focal adhesion kinase silencing via interference RNA suppressed major cytoskeletal proteins synergistically with mineral stressors found in chronic kidney disease in vitro. Conclusions Myocardial failure in advanced chronic kidney disease is characterized by impairment of the cytoskeleton involving disruption of the focal adhesion pathway, mitochondrial failure, and loss of cell survival pathways.
Keywords: cardiovascular; chronic kidney disease; cytoskeleton; dialysis; end stage kidney disease; heart failure; mitochondria.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

