Prospective Evaluation of Coronavirus Disease 2019 (COVID-19) Vaccine Responses Across a Broad Spectrum of Immunocompromising Conditions: the COVID-19 Vaccination in the Immunocompromised Study (COVICS)
- PMID: 35179197
- PMCID: PMC8903515
- DOI: 10.1093/cid/ciac103
Prospective Evaluation of Coronavirus Disease 2019 (COVID-19) Vaccine Responses Across a Broad Spectrum of Immunocompromising Conditions: the COVID-19 Vaccination in the Immunocompromised Study (COVICS)
Abstract
Background: We studied humoral responses after coronavirus disease 2019 (COVID-19) vaccination across varying causes of immunodeficiency.
Methods: Prospective study of fully vaccinated immunocompromised adults (solid organ transplant [SOT], hematologic malignancy, solid cancers, autoimmune conditions, human immunodeficiency virus [HIV]) versus nonimmunocompromised healthcare workers (HCWs). The primary outcome was the proportion with a reactive test (seropositive) for immunoglobulin G to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain. Secondary outcomes were comparisons of antibody levels and their correlation with pseudovirus neutralization titers. Stepwise logistic regression was used to identify factors associated with seropositivity.
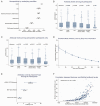
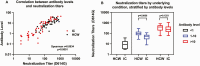
Results: A total of 1271 participants enrolled: 1099 immunocompromised and 172 HCW. Compared with HCW (92.4% seropositive), seropositivity was lower among participants with SOT (30.7%), hematological malignancies (50.0%), autoimmune conditions (79.1%), solid tumors (78.7%), and HIV (79.8%) (P < .01). Factors associated with poor seropositivity included age, greater immunosuppression, time since vaccination, anti-CD20 monoclonal antibodies, and vaccination with BNT162b2 (Pfizer) or adenovirus vector vaccines versus messenger RNA (mRNA)-1273 (Moderna). mRNA-1273 was associated with higher antibody levels than BNT162b2 or adenovirus vector vaccines after adjusting for time since vaccination, age, and underlying condition. Antibody levels were strongly correlated with pseudovirus neutralization titers (Spearman r = 0.89, P < .0001), but in seropositive participants with intermediate antibody levels, neutralization titers were significantly lower in immunocompromised individuals versus HCW.
Conclusions: Antibody responses to COVID-19 vaccines were lowest among SOT and anti-CD20 monoclonal recipients, and recipients of vaccines other than mRNA-1273. Among those with intermediate antibody levels, pseudovirus neutralization titers were lower in immunocompromised patients than HCWs. Additional SARS-CoV-2 preventive approaches are needed for immunocompromised persons, which may need to be tailored to the cause of immunodeficiency.
Keywords: COVID-19 vaccines; SARS-COV-2 antibody; SARS-CoV-2 neutralization; immunocompromised.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

