Association of COVID-19 Acute Respiratory Distress Syndrome With Symptoms of Posttraumatic Stress Disorder in Family Members After ICU Discharge
- PMID: 35179564
- PMCID: PMC8924722
- DOI: 10.1001/jama.2022.2017
Association of COVID-19 Acute Respiratory Distress Syndrome With Symptoms of Posttraumatic Stress Disorder in Family Members After ICU Discharge
Abstract
Importance: Persistent physical and mental disorders are frequent in survivors of COVID-19-related acute respiratory distress syndrome (ARDS). However, data on these disorders among family members are scarce.
Objective: To determine the association between patient hospitalization for COVID-19 ARDS vs ARDS from other causes and the risk of posttraumatic stress disorder (PTSD)-related symptoms in family members.
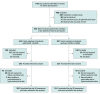
Design, setting, and participants: Prospective cohort study in 23 intensive care units (ICUs) in France (January 2020 to June 2020 with final follow-up ending in October 2020). ARDS survivors and family members (1 family member per patient) were enrolled.
Exposures: Family members of patients hospitalized for ARDS due to COVID-19 vs ARDS due to other causes.
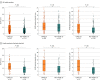
Main outcomes and measures: The primary outcome was family member symptoms of PTSD at 90 days after ICU discharge, measured by the Impact of Events Scale-Revised (score range, 0 [best] to 88 [worst]; presence of PTSD symptoms defined by score >22). Secondary outcomes were family member symptoms of anxiety and depression at 90 days assessed by the Hospital Anxiety and Depression Scale (score range, 0 [best] to 42 [worst]; presence of anxiety or depression symptoms defined by subscale scores ≥7). Multivariable logistic regression models were used to determine the association between COVID-19 status and outcomes.
Results: Among 602 family members and 307 patients prospectively enrolled, 517 (86%) family members (median [IQR] age, 51 [40-63] years; 72% women; 48% spouses; 26% bereaved because of the study patient's death; 303 [50%] family members of COVID-19 patients) and 273 (89%) patients (median [IQR] age, 61 [50-69] years; 34% women; 181 [59%] with COVID-19) completed the day-90 assessment. Compared with non-COVID-19 ARDS, family members of patients with COVID-19 ARDS had a significantly higher prevalence of symptoms of PTSD (35% [103/293] vs 19% [40/211]; difference, 16% [95% CI, 8%-24%]; P < .001), symptoms of anxiety (41% [121/294] vs 34% [70/207]; difference, 8% [95% CI, 0%-16%]; P= .05), and symptoms of depression (31% [91/291] vs 18% [37/209]; difference, 13% [95% CI, 6%-21%]; P< .001). In multivariable models adjusting for age, sex, and level of social support, COVID-19 ARDS was significantly associated with increased risk of PTSD-related symptoms in family members (odds ratio, 2.05 [95% CI, 1.30 to 3.23]).
Conclusions and relevance: Among family members of patients hospitalized in the ICU with ARDS, COVID-19 disease, as compared with other causes of ARDS, was significantly associated with increased risk of symptoms of PTSD at 90 days after ICU discharge.
Trial registration: ClinicalTrials.gov Identifier: NCT04341519.
Conflict of interest statement
Figures

Comment in
-
COVID-19 ARDS and Posttraumatic Stress Disorder in Family Members After ICU Discharge.JAMA. 2022 Jul 19;328(3):301. doi: 10.1001/jama.2022.8792. JAMA. 2022. PMID: 35852533 No abstract available.
-
COVID-19 ARDS and Posttraumatic Stress Disorder in Family Members After ICU Discharge.JAMA. 2022 Jul 19;328(3):301-302. doi: 10.1001/jama.2022.8789. JAMA. 2022. PMID: 35852534 No abstract available.
-
Mental health symptoms are not correlated with peripheral inflammatory biomarkers concentrations in COVID-19-ARDS survivors.Intensive Care Med. 2023 Jan;49(1):109-111. doi: 10.1007/s00134-022-06936-2. Epub 2022 Nov 30. Intensive Care Med. 2023. PMID: 36450929 Free PMC article. No abstract available.

