Efgartigimod: First Approval
- PMID: 35179720
- PMCID: PMC8855644
- DOI: 10.1007/s40265-022-01678-3
Efgartigimod: First Approval
Erratum in
-
Correction to: Efgartigimod: First Approval.Drugs. 2022 Apr;82(5):611. doi: 10.1007/s40265-022-01712-4. Drugs. 2022. PMID: 35353355 Free PMC article. No abstract available.
Abstract
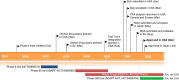
Efgartigimod (efgartigimod alfa-fcab, Vyvgart™) is a first-in-class neonatal Fc receptor antagonist being developed by argenx for the treatment of autoimmune diseases including myasthenia gravis. In December 2021, intravenous efgartigimod received its first approval in the USA for the treatment of generalized myasthenia gravis in adults who are anti-acetylcholine receptor (AChR) antibody positive. Intravenous efgartigimod has also been evaluated for generalized myasthenia gravis in various other countries, with the agent subsequently approved in Japan in January 2022 for generalized myasthenia gravis patients regardless of antibody status and in preregistration stage in the EU. Several clinical studies of intravenous and subcutaneous formulation of efgartigimod are also being investigated for other autoimmune diseases including bullous pemphigoid, chronic inflammatory demyelinating polyradiculoneuropathy, immune thrombocytopenia, autoimmune myositis and pemphigus. This article summarizes the milestones in the development of efgartigimod leading to this first approval for generalized myasthenia gravis.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Young-A Heo is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Figures
References
-
- argenx. FDA approves new treatment for myasthenia gravis [media release]. 17 Dec 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-new-tre....
-
- argenx. argenx announces VYVGART™ approval in Japan for the treatment of generalized myasthenia gravis [media release]. 20 Jan 2022. https://www.argenx.com/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical