Choroidal arteriovenous anastomoses: a hypothesis for the pathogenesis of central serous chorioretinopathy and other pachychoroid disease spectrum abnormalities
- PMID: 35179828
- PMCID: PMC9790326
- DOI: 10.1111/aos.15112
Choroidal arteriovenous anastomoses: a hypothesis for the pathogenesis of central serous chorioretinopathy and other pachychoroid disease spectrum abnormalities
Abstract
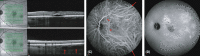
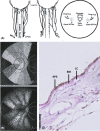
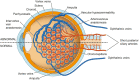
The pachychoroid disease spectrum (PDS) includes several chorioretinal diseases that share specific choroidal abnormalities. Although their pathophysiological basis is poorly understood, diseases that are part of the PDS have been hypothesized to be the result of venous congestion. Within the PDS, central serous chorioretinopathy is the most common condition associated with vision loss, due to an accumulation of subretinal fluid in the macula. Central serous chorioretinopathy is characterized by distinct risk factors, most notably a high prevalence in males and exposure to corticosteroids. Interestingly, sex differences and corticosteroids are also strongly associated with specific types of arteriovenous anastomoses in the human body, including dural arteriovenous fistula and surgically created arteriovenous shunts. In this manuscript, we assess the potential of such arteriovenous anastomoses in the choroid as a causal mechanism of the PDS. We propose how this may provide a novel unifying concept on the pathophysiological basis of the PDS, and present cases in which this mechanism may play a role.
Keywords: arteriovenous anastomoses; arteriovenous fistula; corticosteroids; pachychoroid disease spectrum; sex differences; venous congestion.
© 2022 The Authors. Acta Ophthalmologica published by John Wiley & Sons Ltd on behalf of Acta Ophthalmologica Scandinavica Foundation.
Figures


References
-
- Amyere M, Revencu N, Helaers R et al. (2017): Germline loss‐of‐function mutations in EPHB4 cause a second form of capillary malformation‐arteriovenous malformation (CM‐AVM2) deregulating RAS‐MAPK signaling. Circulation 136: 1037–1048. - PubMed
-
- Baek J, Dansingani KK, Lee JH, Lee WK & Freund KB (2019a): Choroidal morphology in eyes with peripapillary polypoidal choroidal vasculopathy. Retina 39: 1571–1579. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

