Stable and Functionally Diverse Versatile Peroxidases Designed Directly from Sequences
- PMID: 35179866
- PMCID: PMC8895400
- DOI: 10.1021/jacs.1c12433
Stable and Functionally Diverse Versatile Peroxidases Designed Directly from Sequences
Abstract
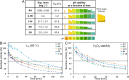
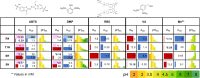
White-rot fungi secrete a repertoire of high-redox potential oxidoreductases to efficiently decompose lignin. Of these enzymes, versatile peroxidases (VPs) are the most promiscuous biocatalysts. VPs are attractive enzymes for research and industrial use but their recombinant production is extremely challenging. To date, only a single VP has been structurally characterized and optimized for recombinant functional expression, stability, and activity. Computational enzyme optimization methods can be applied to many enzymes in parallel but they require accurate structures. Here, we demonstrate that model structures computed by deep-learning-based ab initio structure prediction methods are reliable starting points for one-shot PROSS stability-design calculations. Four designed VPs encoding as many as 43 mutations relative to the wildtype enzymes are functionally expressed in yeast, whereas their wildtype parents are not. Three of these designs exhibit substantial and useful diversity in their reactivity profiles and tolerance to environmental conditions. The reliability of the new generation of structure predictors and design methods increases the scale and scope of computational enzyme optimization, enabling efficient discovery and exploitation of the functional diversity in natural enzyme families directly from genomic databases.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

