Interaction with a Biomolecule Facilitates the Formation of the Function-Determining Long-Lived Triplet State in a Ruthenium Complex for Photodynamic Therapy
- PMID: 35179905
- PMCID: PMC8903189
- DOI: 10.1021/acs.jpca.1c09968
Interaction with a Biomolecule Facilitates the Formation of the Function-Determining Long-Lived Triplet State in a Ruthenium Complex for Photodynamic Therapy
Abstract
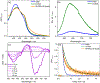
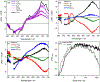
TLD1433 is the first ruthenium (Ru)-based photodynamic therapy (PDT) agent to advance to clinical trials and is currently in a phase II study for treating nonmuscle bladder cancer with PDT. Herein, we present a photophysical study of TLD1433 and its derivative TLD1633 using complex, biologically relevant solvents to elucidate the excited-state properties that are key for biological activity. The complexes incorporate an imidazo [4,5-f][1,10]phenanthroline (IP) ligand appended to α-ter- or quaterthiophene, respectively, where TLD1433 = [Ru(4,4'-dmb)2(IP-3T)]Cl2 and TLD1633 = [Ru(4,4'-dmb)2(IP-4T)]Cl2 (4,4'-dmb = 4,4'-dimethyl-2,2'-bipyridine; 3T = α-terthiophene; 4T = α-quaterthiophene). Time-resolved transient absorption experiments demonstrate that the excited-state dynamics of the complexes change upon interaction with biological macromolecules (e.g., DNA). In this case, the accessibility of the lowest-energy triplet intraligand charge-transfer (3ILCT) state (T1) is increased at the expense of a higher-lying 3ILCT state. We attribute this behavior to the increased rigidity of the ligand framework upon binding to DNA, which prolongs the lifetime of the T1 state. This lowest-lying state is primarily responsible for O2 sensitization and hence photoinduced cytotoxicity. Therefore, to gain a realistic picture of the excited-state kinetics that underlie the photoinduced function of the complexes, it is necessary to interrogate their photophysical dynamics in the presence of biological targets once they are known.
Conflict of interest statement
S.A.M. has a potential research conflict of interest due to a financial interest with Theralase Technologies, Inc. and PhotoDynamic, Inc. A management plan has been created to preserve objectivity in research in accordance with UTA policy
Figures
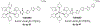

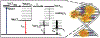
References
-
- Bonnett R Chemical Aspects of Photodynamic Therapy; CRC Press: London, 2000.
-
- Photodynamic Therapy: Basic Principles and Clinical Applications; Henderson BW, Dougherty TJ, Eds.; CRC Press: New York, 1992
-
- Handbook of Photomedicine, 1st ed.; Hamblin MR, Huang Y, Eds.; CRC Press: Boca Raton, FL, 2013.
-
- Hamblin MR; Mroz P Advances in Photodynamic Therapy: Basic, Translational, and Clinical; Engineering in Medicine and Biology; Artech House: Norwood, MA, 2008.
-
- Photodynamic Medicine: From Bench to Clinic, 1st ed.; Kostron H, Hasan T, Eds.; Royal Society of Chemistry: Cambridge, U.K., 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

