COVID-19 vaccination and Guillain-Barré syndrome: analyses using the National Immunoglobulin Database
- PMID: 35180300
- PMCID: PMC8903477
- DOI: 10.1093/brain/awac067
COVID-19 vaccination and Guillain-Barré syndrome: analyses using the National Immunoglobulin Database
Abstract
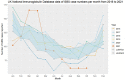
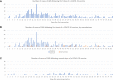
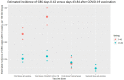
Vaccination against viruses has rarely been associated with Guillain-Barré syndrome (GBS), and an association with the COVID-19 vaccine is unknown. We performed a population-based study of National Health Service data in England and a multicentre surveillance study from UK hospitals to investigate the relationship between COVID-19 vaccination and GBS. Firstly, case dates of GBS identified retrospectively in the National Immunoglobulin Database from 8 December 2021 to 8 July 2021 were linked to receipt dates of COVID-19 vaccines using data from the National Immunisation Management System in England. For the linked dataset, GBS cases temporally associated with vaccination within a 6-week risk window of any COVID-19 vaccine were identified. Secondly, we prospectively collected incident UK-wide (four nations) GBS cases from 1 January 2021 to 7 November 2021 in a separate UK multicentre surveillance database. For this multicentre UK-wide surveillance dataset, we explored phenotypes of reported GBS cases to identify features of COVID-19 vaccine-associated GBS. Nine hundred and ninety-six GBS cases were recorded in the National Immunoglobulin Database from January to October 2021. A spike of GBS cases above the 2016-2020 average occurred in March-April 2021. One hundred and ninety-eight GBS cases occurred within 6 weeks of the first-dose COVID-19 vaccination in England [0.618 cases per 100,000 vaccinations; 176 ChAdOx1 nCoV-19 (AstraZeneca), 21 tozinameran (Pfizer) and one mRNA-1273 (Moderna)]. The 6-week excess of GBS (compared to the baseline rate of GBS cases 6-12 weeks after vaccination) occurred with a peak at 24 days post-vaccination; first-doses of ChAdOx1 nCoV-19 accounted for the excess. No excess was seen for second-dose vaccination. The absolute number of excess GBS cases from January-July 2021 was between 98-140 cases for first-dose ChAdOx1 nCoV-19 vaccination. First-dose tozinameran and second-dose of any vaccination showed no excess GBS risk. Detailed clinical data from 121 GBS patients were reported in the separate multicentre surveillance dataset during this timeframe. No phenotypic or demographic differences identified between vaccine-associated and non-vaccinated GBS cases occurring in the same timeframe. Analysis of the linked NID/NIMS dataset suggested that first-dose ChAdOx1 nCoV-19 vaccination is associated with an excess GBS risk of 0.576 (95% confidence interval 0.481-0.691) cases per 100 000 doses. However, examination of a multicentre surveillance dataset suggested that no specific clinical features, including facial weakness, are associated with vaccination-related GBS compared to non-vaccinated cases. The pathogenic cause of the ChAdOx1 nCoV-19 specific first dose link warrants further study.
Keywords: COVID-19 vaccination; Guillain-Barré syndrome.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Rogers JP, Watson CJ, Badenoch J, et al. . Neurology and neuropsychiatry of COVID-19: a systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J Neurol Neurosurg Psychiatry. 2021;92(9):932–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

