Forging Forward in Photodynamic Therapy
- PMID: 35180305
- PMCID: PMC11855711
- DOI: 10.1158/0008-5472.CAN-21-4122
Forging Forward in Photodynamic Therapy
Abstract
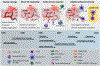
In 1978, a Cancer Research article by Dougherty and colleagues reported the first large-scale clinical trial of photodynamic therapy (PDT) for treatment of 113 cutaneous or subcutaneous lesions associated with ten different kinds of malignancies. In classic applications, PDT depends on excitation of a tissue-localized photosensitizer with wavelengths of visible light to damage malignant or otherwise diseased tissues. Thus, in this landmark article, photosensitizer (hematoporphyrin derivative) dose, drug-light interval, and fractionation scheme were evaluated for their therapeutic efficacy and normal tissue damage. From their observations came early evidence of the mechanisms of PDT's antitumor action, and in the decades since this work, our knowledge of these mechanisms has grown to build an understanding of the multifaceted nature of PDT. These facets are comprised of multiple cell death pathways, together with antivascular and immune stimulatory actions that constitute a PDT reaction. Mechanism-informed PDT protocols support the contribution of PDT to multimodality treatment approaches. Moreover, guided by an understanding of its mechanisms, PDT can be applied to clinical needs in fields beyond oncology. Undoubtedly, there still remains more to learn; new modes of cell death continue to be elucidated with relevance to PDT, and factors that drive PDT innate and adaptive immune responses are not yet fully understood. As research continues to forge a path forward for PDT in the clinic, direction is provided by anchoring new applications in mechanistically grounded protocol design, as was first exemplified in the landmark work conducted by Dougherty and colleagues. See related article by Dougherty and colleagues, Cancer Res 1978;38:2628-35.
©2022 American Association for Cancer Research.
Figures
References
-
- Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res 1978;38:2628–35. - PubMed
-
- Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer 2019;1872:188308. - PubMed
-
- Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol 2020;17:657–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

