Modeling apoptosis resistance in CHO cells with CRISPR-mediated knockouts of Bak1, Bax, and Bok
- PMID: 35180317
- PMCID: PMC9310834
- DOI: 10.1002/bit.28062
Modeling apoptosis resistance in CHO cells with CRISPR-mediated knockouts of Bak1, Bax, and Bok
Abstract
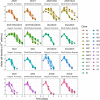
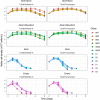
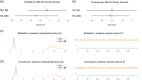
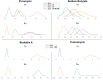
Chinese hamster ovary (CHO) cells are the primary platform for the production of biopharmaceuticals. To increase yields, many CHO cell lines have been genetically engineered to resist cell death. However, the kinetics that governs cell fate in bioreactors are confounded by many variables associated with batch processes. Here, we used CRISPR-Cas9 to create combinatorial knockouts of the three known BCL-2 family effector proteins: Bak1, Bax, and Bok. To assess the response to apoptotic stimuli, cell lines were cultured in the presence of four cytotoxic compounds with different mechanisms of action. A population-based model was developed to describe the behavior of the resulting viable cell dynamics as a function of genotype and treatment. Our results validated the synergistic antiapoptotic nature of Bak1 and Bax, while the deletion of Bok had no significant impact. Importantly, the uniform application of apoptotic stresses permitted direct observation and quantification of a delay in the onset of cell death through Bayesian inference of meaningful model parameters. In addition to the classical death rate, a delay function was found to be essential in the accurate modeling of the cell death response. These findings represent an important bridge between cell line engineering strategies and biological modeling in a bioprocess context.
Keywords: Bayesian inference; CHO cells; CRISPR; apoptosis; bioprocessing; population model.
© 2022 The Authors. Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Figures



References
-
- Canzler, S. , Schor, J. , Busch, W. , Schubert, K. , Rolle‐Kampczyk, U. E. , Seitz, H. , Kamp, H. , von Bergen, M. , Buesen, R. , & Hackermüller, J. (2020). Prospects and challenges of multi‐omics data integration in toxicology. Archives of Toxicology, 94(2), 371–388. 10.1007/s00204-020-02656-y - DOI - PubMed
-
- Chen, F. , Kou, T. C. , Fan, L. , Zhou, Y. , Ye, Z. Y. , Zhao, L. , & Tan, W. S. (2011). The combined effect of sodium butyrate and low culture temperature on the production, sialylation, and biological activity of an antibody produced in CHO cells. Biotechnology and Bioprocess Engineering, 16(6), 1157–1165. 10.1007/s12257-011-0069-8 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

