Differential dendritic integration of long-range inputs in association cortex via subcellular changes in synaptic AMPA-to-NMDA receptor ratio
- PMID: 35180389
- PMCID: PMC9081173
- DOI: 10.1016/j.neuron.2022.01.025
Differential dendritic integration of long-range inputs in association cortex via subcellular changes in synaptic AMPA-to-NMDA receptor ratio
Abstract
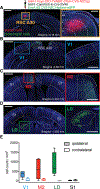
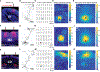
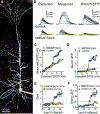
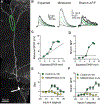
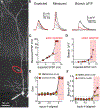
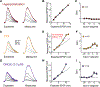
Synaptic NMDA receptors can produce powerful dendritic supralinearities that expand the computational repertoire of single neurons and their respective circuits. This form of supralinearity may represent a general principle for synaptic integration in thin dendrites. However, individual cortical neurons receive many diverse classes of input that may require distinct postsynaptic decoding schemes. Here, we show that sensory, motor, and thalamic inputs preferentially target basal, apical oblique, and distal tuft dendrites, respectively, in layer 5b pyramidal neurons of the mouse retrosplenial cortex, a visuospatial association area. These dendritic compartments exhibited differential expression of NMDA receptor-mediated supralinearity due to systematic changes in the AMPA-to-NMDA receptor ratio. Our results reveal a new schema for integration in cortical pyramidal neurons, in which dendrite-specific changes in synaptic receptors support input-localized decoding. This coexistence of multiple modes of dendritic integration in single neurons has important implications for synaptic plasticity and cortical computation.
Keywords: AMPA; L5b pyramidal cells; NMDA; dendritic integration; retrosplenial cortex; sCRACM.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
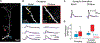
Comment in
-
Connecting axons and dendrites: An oblique view.Neuron. 2022 May 4;110(9):1438-1440. doi: 10.1016/j.neuron.2022.04.014. Neuron. 2022. PMID: 35512635
References
-
- Ariav G, Polsky A and Schiller J (2003) ‘Submillisecond precision of the input-output transformation function mediated by fast sodium dendritic spikes in basal dendrites of CA1 pyramidal neurons’, Journal of Neuroscience, 23(21), pp. 7750–7758. doi: 10.1523/jneurosci.23-21-07750.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

