ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury
- PMID: 35180475
- PMCID: PMC8857079
- DOI: 10.1016/j.redox.2022.102262
ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury
Abstract
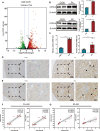
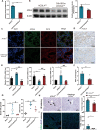
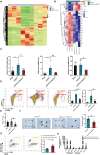
The term ferroptosis coined in 2012 causes acute kidney injury (AKI). However, its pathway mechanism in AKI is poorly understood. In this study, we conducted an RNA-sequence analysis of kidneys in AKI and normal mice to explore the pathway mechanism of ferroptosis. Consequently, differentially expressed genes highlighted Acyl-CoA synthetase long-chain family (ACSL4), a known promotor for ferroptosis. Besides, RT-PCR, Western blot, and immunohistochemical analyses confirmed its upregulation. HIF-1α was downregulated in I/R-AKI mice, and in vitro studies confirmed a negative regulation of HIF-1α on ACSL4. To explore the role of ACSL4 in AKI, we constructed ACSL4 knockout in kidney tubules of mice-as Cdh16Cre-ACSL4F/F mice. Results revealed that ACSL4 knockout significantly reduced ferroptosis and inhibited the functional and pathological injury of AKI mice. Meanwhile, the kidneys of Cdh16Cre-ACSL4F/F mice demonstrated a significantly decreased inflammation and macrophage infiltration. Further, additional explorations were explored to decipher a more thorough understanding of ferroptotic immunogenicity. As a result, neutrophils were not directly recruited by ferroptotic cells, but by ferroptotic cell-induced macrophages. Further, ACSL4 inhibitor rosiglitazone significantly inhibited AKI. Collectively, these data provide novel insights into the AKI pathogenesis, and defined ACSL4 as an effective target in AKI.
Keywords: ACSL4; Acute kidney injury; Ferroptosis; HIF-1α; Macrophages.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Lameire N.H., Bagga A., Cruz D., et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. - PubMed
-
- Levey A.S., James M.T. Acute kidney injury. Ann. Intern. Med. 2017;167:ITC66–ITC80. - PubMed
-
- Pannu N., Nadim M.K. An overview of drug-induced acute kidney injury. Crit. Care Med. 2008;36:S216–S223. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

