Mass cytometry reveals single-cell kinetics of cytotoxic lymphocyte evolution in CMV-infected renal transplant patients
- PMID: 35181606
- PMCID: PMC8872722
- DOI: 10.1073/pnas.2116588119
Mass cytometry reveals single-cell kinetics of cytotoxic lymphocyte evolution in CMV-infected renal transplant patients
Abstract
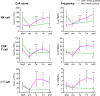
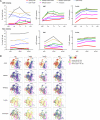
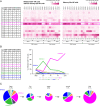
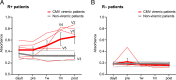
Cytomegalovirus (CMV) infection is associated with graft rejection in renal transplantation. Memory-like natural killer (NK) cells expressing NKG2C and lacking FcεRIγ are established during CMV infection. Additionally, CD8+ T cells expressing NKG2C have been observed in some CMV-seropositive patients. However, in vivo kinetics detailing the development and differentiation of these lymphocyte subsets during CMV infection remain limited. Here, we interrogated the in vivo kinetics of lymphocytes in CMV-infected renal transplant patients using longitudinal samples compared with those of nonviremic (NV) patients. Recipient CMV-seropositive (R+) patients had preexisting memory-like NK cells (NKG2C+CD57+FcεRIγ-) at baseline, which decreased in the periphery immediately after transplantation in both viremic and NV patients. We identified a subset of prememory-like NK cells (NKG2C+CD57+FcεRIγlow-dim) that increased during viremia in R+ viremic patients. These cells showed a higher cytotoxic profile than preexisting memory-like NK cells with transient up-regulation of FcεRIγ and Ki67 expression at the acute phase, with the subsequent accumulation of new memory-like NK cells at later phases of viremia. Furthermore, cytotoxic NKG2C+CD8+ T cells and γδ T cells significantly increased in viremic patients but not in NV patients. These three different cytotoxic cells combinatorially responded to viremia, showing a relatively early response in R+ viremic patients compared with recipient CMV-seronegative viremic patients. All viremic patients, except one, overcame viremia and did not experience graft rejection. These data provide insights into the in vivo dynamics and interplay of cytotoxic lymphocytes responding to CMV viremia, which are potentially linked with control of CMV viremia to prevent graft rejection.
Keywords: NK cell; cytomegalovirus; cytotoxic lymphocyte; mass cytometry; renal transplantation.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Dzabic M., et al. , Intragraft cytomegalovirus protein expression is associated with reduced renal allograft survival. Clin. Infect. Dis. 53, 969–976 (2011). - PubMed
-
- Gatault P., et al. , CMV infection in the donor and increased kidney graft loss: Impact of full HLA-I mismatch and posttransplantation CD8(+) cell reduction. Am. J. Transplant. 13, 2119–2129 (2013). - PubMed

