Mechanical regulation of bone remodeling
- PMID: 35181672
- PMCID: PMC8857305
- DOI: 10.1038/s41413-022-00190-4
Mechanical regulation of bone remodeling
Abstract
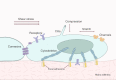
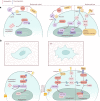
Bone remodeling is a lifelong process that gives rise to a mature, dynamic bone structure via a balance between bone formation by osteoblasts and resorption by osteoclasts. These opposite processes allow the accommodation of bones to dynamic mechanical forces, altering bone mass in response to changing conditions. Mechanical forces are indispensable for bone homeostasis; skeletal formation, resorption, and adaptation are dependent on mechanical signals, and loss of mechanical stimulation can therefore significantly weaken the bone structure, causing disuse osteoporosis and increasing the risk of fracture. The exact mechanisms by which the body senses and transduces mechanical forces to regulate bone remodeling have long been an active area of study among researchers and clinicians. Such research will lead to a deeper understanding of bone disorders and identify new strategies for skeletal rejuvenation. Here, we will discuss the mechanical properties, mechanosensitive cell populations, and mechanotransducive signaling pathways of the skeletal system.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Wolff JDas. Gesetz der transformation der Knochen. A Hirshwald. 1892;1:1–152.
-
- Frost HM. The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs. J. Bone Miner. Metab. 2000;18:305–316. - PubMed
-
- Nicogossian A. Medicine and space exploration. Lancet. 2003;362:s8–s9. - PubMed
-
- Vico L, Hargens A. Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 2018;14:229–245. - PubMed
-
- Takata S, Yasui N. Disuse osteoporosis. J. Med. Investig. 2001;48:147–156. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

