Holistic Characterization of Tumor Monocyte-to-Macrophage Differentiation Integrates Distinct Immune Phenotypes in Kidney Cancer
- PMID: 35181780
- PMCID: PMC8982148
- DOI: 10.1158/2326-6066.CIR-21-0588
Holistic Characterization of Tumor Monocyte-to-Macrophage Differentiation Integrates Distinct Immune Phenotypes in Kidney Cancer
Abstract
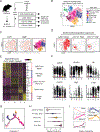
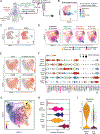
The tumor immune microenvironment (TIME) is commonly infiltrated by diverse collections of myeloid cells. Yet, the complexity of myeloid-cell identity and plasticity has challenged efforts to define bona fide populations and determine their connections to T-cell function and their relationship to patient outcome. Here, we have leveraged single-cell RNA-sequencing analysis of several mouse and human tumors and found that monocyte-macrophage diversity is characterized by a combination of conserved lineage states as well as transcriptional programs accessed along the differentiation trajectory. We also found in mouse models that tumor monocyte-to-macrophage progression was profoundly tied to regulatory T cell (Treg) abundance. In human kidney cancer, heterogeneity in macrophage accumulation and myeloid composition corresponded to variance in, not only Treg density, but also the quality of infiltrating CD8+ T cells. In this way, holistic analysis of monocyte-to-macrophage differentiation creates a framework for critically different immune states.
©2022 American Association for Cancer Research.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

