Choroidal endothelial and macrophage gene expression in atrophic and neovascular macular degeneration
- PMID: 35181781
- PMCID: PMC9307320
- DOI: 10.1093/hmg/ddac043
Choroidal endothelial and macrophage gene expression in atrophic and neovascular macular degeneration
Abstract
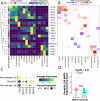
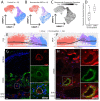
The human choroid is a heterogeneous, highly vascular connective tissue that dysfunctions in age-related macular degeneration (AMD). In this study, we performed single-cell RNA sequencing on 21 human choroids, 11 of which were derived from donors with early atrophic or neovascular AMD. Using this large donor cohort, we identified new gene expression signatures and immunohistochemically characterized discrete populations of resident macrophages, monocytes/inflammatory macrophages and dendritic cells. These three immune populations demonstrated unique expression patterns for AMD genetic risk factors, with dendritic cells possessing the highest expression of the neovascular AMD-associated MMP9 gene. Additionally, we performed trajectory analysis to model transcriptomic changes across the choroidal vasculature, and we identified expression signatures for endothelial cells from choroidal arterioles and venules. Finally, we performed differential expression analysis between control, early atrophic AMD, and neovascular AMD samples, and we observed that early atrophic AMD samples had high expression of SPARCL1, a gene that has been shown to increase in response to endothelial damage. Likewise, neovascular endothelial cells harbored gene expression changes consistent with endothelial cell damage and demonstrated increased expression of the sialomucins CD34 and ENCM, which were also observed at the protein level within neovascular membranes. Overall, this study characterizes the molecular features of new populations of choroidal endothelial cells and mononuclear phagocytes in a large cohort of AMD and control human donors.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures






References
-
- Hayreh, S.S. (1974) Vascular pattern of the choriocapillaris. Exp. Eye Res., 19, 101–104. - PubMed
-
- Biesemeier, A., Taubitz, T., Julien, S., Yoeruek, E. and Schraermeyer, U. (2014) Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol. Aging, 35, 2562–2573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

