Renin-angiotensin system inhibitors combined with cisplatin exacerbate cisplatin-induced nephrotoxicity in mice
- PMID: 35182957
- PMCID: PMC8857575
- DOI: 10.1016/j.tranon.2022.101369
Renin-angiotensin system inhibitors combined with cisplatin exacerbate cisplatin-induced nephrotoxicity in mice
Abstract
Introduction: We previously reported that the concomitant use of enalapril and telmisartan exacerbates the risk of cisplatin (CDDP)-induced acute renal dysfunction compared to other antihypertensive drugs in mice. Thus, in the current study, we investigated the risk of developing chronic kidney disease following repeated concomitant use of CDDP and antihypertensive drugs.
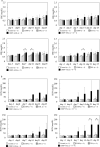
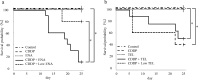
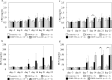
Materials and methods: Male BALB/c mice were divided into 12 groups: (1) Control group (untreated), (2) CDDP group (7 mg/kg, CDDP), (3) AML group (5 mg/kg, amlodipine), (4) ENA group (2.5 mg/kg, enalapril), (5) TEL group (10 mg/kg, telmisartan), (6) LOS group (10 mg/kg, losartan), (7) CDDP+AML group (5 mg/mL, AML), (8) CDDP+ENA group (2.5 mg/kg, ENA), (9) CDDP+LowENA group (1.25 mg/kg, ENA), (10) CDDP+TEL group (10 mg/kg, TEL), (11) CDDP+LowTEL group (5 mg/kg, TEL), and (12) CDDP+LOS group (10 mg/kg, LOS). CDDP was administered intraperitoneally four times every 7 days, and each antihypertensive drug was administered orally from day 3 before CDDP administration until day 24 (six times a week). The degree of renal damage was assessed. The nephrotoxicity of each individual was evaluated by measuring serum creatinine and blood urea nitrogen levels. The degrees of renal fibrosis and epithelial-mesenchymal transition were also examined in kidney tissue sections.
Results and discussion: The results suggest that combinatorial treatment of CDDP and renin-angiotensin system inhibitors, particularly ENA and TEL, may exacerbate CDDP-induced nephrotoxicity. This study clearly demonstrates the need for large-scale clinical studies to construct treatment regimens that do not interfere with the therapeutic intensity of CDDP.
Keywords: Cisplatin; Fibrosis; Nephrotoxicity; Renin-angiotensin system inhibitor.
Copyright © 2022. Published by Elsevier Inc.
Figures



References
-
- de Jongh F.E., van Veen R.N., Veltman S.J., de Wit R., van der Burg M.E., van den Bent M.J., Planting A.S., Graveland W.J., Stoter G., Verweij J. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br. J. Cancer. 2003;88:1199–1206. doi: 10.1038/sj.bjc.6600884. - DOI - PMC - PubMed
-
- Horinouchi H., Kubota K., Itani H., Taniyama T., Nakamichi S., Wakui H., Kanda S., Nokihara H., Yamamoto N., Sekine I., Tamura T. Short hydration in chemotherapy containing cisplatin (≥75 mg/m2) for patients with lung cancer: a prospective study. Jpn. J. Clin. Oncol. 2013;43:1105–1109. doi: 10.1093/jjco/hyt122. - DOI - PubMed
LinkOut - more resources
Full Text Sources

