Lansoprazole-induced osteoporosis via the IP3R- and SOCE-mediated calcium signaling pathways
- PMID: 35183103
- PMCID: PMC8858482
- DOI: 10.1186/s10020-022-00448-x
Lansoprazole-induced osteoporosis via the IP3R- and SOCE-mediated calcium signaling pathways
Abstract
Background: Many clinical studies have shown a correlation between proton pump inhibitors (PPIs) and osteoporosis or fractures. The purpose of this study was to establish a murine model of chronic oral PPI administration to verify whether PPIs caused bone metabolic impairment and investigate the relevant molecular mechanism underlying the effects of PPIs on MC3T3-E1 murine osteoblasts.
Methods: A lansoprazole-induced bone loss model was used to investigate the damaging effects of PPIs. In vivo, immunohistochemistry, Hematoxylin-Eosin (HE) staining, micro-CT analysis, and blood biochemical analyses were used to evaluate the effect of lansoprazole on bone injury in mice. In vitro, the effects of lansoprazole and related signaling pathways in MC3T3-E1 cells were investigated by CCK-8 assays, EdU assays, flow cytometry, laser confocal microscopy, patch clamping, reverse transcription-quantitative polymerase chain reaction and Western blotting.
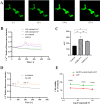
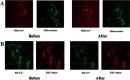
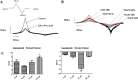
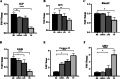
Results: After 6 months of lansoprazole gavage in ICR mice, the micro-CT results showed that compared with that in the vehicle group, the bone mineral density (BMD) in the high-dose group was significantly decreased (P < 0.05), and the bone microarchitecture gradually degraded. Biochemical analysis of bone serum showed that blood calcium and phosphorus were both decreased (P < 0.01). We found that long-term administration of lansoprazole impaired skeletal function in mice. In vitro, we found that lansoprazole (LPZ) could cause calcium overload in MC3T3-E1 cells leading to apoptosis, and 2-APB, an inhibitor of IP3R calcium release channel and SOCE pathway, effectively blocked increase in calcium caused by LPZ, thus protecting cell viability.
Conclusions: Longterm administration of LPZ induced osteoporotic symptoms in mice, and LPZ triggered calcium increases in osteoblasts in a concentration-dependent manner. Intracellular calcium ([Ca2+]i) persisted at a high concentration, thereby causing endoplasmic reticulum stress (ERS) and inducing osteoblast apoptosis.
Keywords: Calcium overload; ER stress; IP3R; Lansoprazole; Osteoporosis; SOCE.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures










Similar articles
-
Lansoprazole promotes cisplatin-induced acute kidney injury via enhancing tubular necroptosis.J Cell Mol Med. 2021 Mar;25(5):2703-2713. doi: 10.1111/jcmm.16302. Epub 2021 Feb 18. J Cell Mol Med. 2021. PMID: 33605079 Free PMC article.
-
Effects of JSOG-6 on protection against bone loss in ovariectomized mice through regulation of osteoblast differentiation and osteoclast formation.BMC Complement Altern Med. 2014 Jun 6;14:184. doi: 10.1186/1472-6882-14-184. BMC Complement Altern Med. 2014. PMID: 24903150 Free PMC article.
-
Gastrodin protects MC3T3-E1 osteoblasts from dexamethasone-induced cellular dysfunction and promotes bone formation via induction of the NRF2 signaling pathway.Int J Mol Med. 2018 Apr;41(4):2059-2069. doi: 10.3892/ijmm.2018.3414. Epub 2018 Jan 23. Int J Mol Med. 2018. PMID: 29393365 Free PMC article.
-
[Proton-pump inhibitor use and hyponatremia].Nihon Ronen Igakkai Zasshi. 2023;60(2):153-157. doi: 10.3143/geriatrics.60.153. Nihon Ronen Igakkai Zasshi. 2023. PMID: 37225507 Japanese.
-
Strontium ranelate in osteoporosis.Curr Pharm Des. 2002;8(21):1907-16. doi: 10.2174/1381612023393639. Curr Pharm Des. 2002. PMID: 12171530 Review.
Cited by
-
Sex-specific Association of Chronic Proton Pump Inhibitor Use With Reduced Bone Density and Quality.J Clin Endocrinol Metab. 2025 May 19;110(6):e2071-e2079. doi: 10.1210/clinem/dgae598. J Clin Endocrinol Metab. 2025. PMID: 39197024 Free PMC article.
-
The role of calcium channels in osteoporosis and their therapeutic potential.Front Endocrinol (Lausanne). 2024 Aug 7;15:1450328. doi: 10.3389/fendo.2024.1450328. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39170742 Free PMC article. Review.
-
Lead (Pb) Induces Osteotoxicity Through the Activation of Mutually Reinforced ER Stress and ROS in MC3T3-E1 Cells.Biol Trace Elem Res. 2025 Jul;203(7):3820-3832. doi: 10.1007/s12011-024-04427-7. Epub 2024 Dec 6. Biol Trace Elem Res. 2025. PMID: 39643796
-
Clinical Use of Lansoprazole and the Risk of Osteoporosis: A Nationwide Cohort Study.Int J Environ Res Public Health. 2022 Nov 21;19(22):15359. doi: 10.3390/ijerph192215359. Int J Environ Res Public Health. 2022. PMID: 36430077 Free PMC article.
-
Endoplasmic reticulum stress: a novel targeted approach to repair bone defects by regulating osteogenesis and angiogenesis.J Transl Med. 2023 Jul 18;21(1):480. doi: 10.1186/s12967-023-04328-8. J Transl Med. 2023. PMID: 37464413 Free PMC article. Review.
References
-
- Chen J, Li L, Bai X, Xiao L, Shangguan J, Zhang W, et al. Inhibition of autophagy prevents panax notoginseng saponins (PNS) protection on cardiac myocytes against endoplasmic reticulum (ER) stress-induced mitochondrial injury, Ca(2+) homeostasis and associated apoptosis. Front Pharmacol. 2021;12:620812. doi: 10.3389/fphar.2021.620812. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

