Exploring neuronal mechanisms involved in the scratching behavior of a mouse model of allergic contact dermatitis by transcriptomics
- PMID: 35183104
- PMCID: PMC8903649
- DOI: 10.1186/s11658-022-00316-w
Exploring neuronal mechanisms involved in the scratching behavior of a mouse model of allergic contact dermatitis by transcriptomics
Abstract
Background: Allergic contact dermatitis (ACD) is a common skin condition characterized by contact hypersensitivity to allergens, accompanied with skin inflammation and a mixed itch and pain sensation. The itch and pain dramatically affects patients' quality of life. However, still little is known about the mechanisms triggering pain and itch sensations in ACD.
Methods: We established a mouse model of ACD by sensitization and repetitive challenge with the hapten oxazolone. Skin pathological analysis, transcriptome RNA sequencing (RNA-seq), qPCR, Ca2+ imaging, immunostaining, and behavioral assay were used for identifying gene expression changes in dorsal root ganglion innervating the inflamed skin of ACD model mice and for further functional validations.
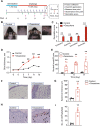
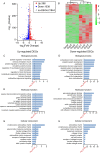
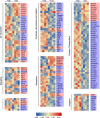
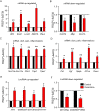
Results: The model mice developed typical ACD symptoms, including skin dryness, erythema, excoriation, edema, epidermal hyperplasia, inflammatory cell infiltration, and scratching behavior, accompanied with development of eczematous lesions. Transcriptome RNA-seq revealed a number of differentially expressed genes (DEGs), including 1436-DEG mRNAs and 374-DEG-long noncoding RNAs (lncRNAs). We identified a number of DEGs specifically related to sensory neuron signal transduction, pain, itch, and neuroinflammation. Comparison of our dataset with another published dataset of atopic dermatitis mouse model identified a core set of genes in peripheral sensory neurons that are exclusively affected by local skin inflammation. We further found that the expression of the pain and itch receptor MrgprD was functionally upregulated in dorsal root ganglia (DRG) neurons innervating the inflamed skin of ACD model mice. MrgprD activation induced by its agonist β-alanine resulted in exaggerated scratching responses in ACD model mice compared with naïve mice.
Conclusions: We identified the molecular changes and cellular pathways in peripheral sensory ganglia during ACD that might participate in neurogenic inflammation, pain, and itch. We further revealed that the pain and itch receptor MrgprD is functionally upregulated in DRG neurons, which might contribute to peripheral pain and itch sensitization during ACD. Thus, targeting MrgprD may be an effective method for alleviating itch and pain in ACD.
Keywords: Allergic contact dermatitis; Itch; Pain; RNA-seq; Sensory neurons.
© 2022. The Author(s).
Conflict of interest statement
No competing interest was declared.
Figures
Similar articles
-
Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain.Brain. 2014 Apr;137(Pt 4):1039-50. doi: 10.1093/brain/awu007. Epub 2014 Feb 18. Brain. 2014. PMID: 24549959 Free PMC article.
-
IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):E7572-E7579. doi: 10.1073/pnas.1606608113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821781 Free PMC article.
-
CCL2/CCR2 signaling elicits itch- and pain-like behavior in a murine model of allergic contact dermatitis.Brain Behav Immun. 2019 Aug;80:464-473. doi: 10.1016/j.bbi.2019.04.026. Epub 2019 Apr 11. Brain Behav Immun. 2019. PMID: 30981714
-
The Unique Molecular Signatures of Contact Dermatitis and Implications for Treatment.Clin Rev Allergy Immunol. 2019 Feb;56(1):1-8. doi: 10.1007/s12016-018-8685-0. Clin Rev Allergy Immunol. 2019. PMID: 29754191 Review.
-
Peripheral itch sensitization in atopic dermatitis.Allergol Int. 2022 Jul;71(3):265-277. doi: 10.1016/j.alit.2022.04.003. Epub 2022 May 25. Allergol Int. 2022. PMID: 35624035 Review.
Cited by
-
In-vitro quantitative measurement and analysis of the photosensitivity of cells to a weak pulse laser.Biomed Opt Express. 2023 Jun 23;14(7):3584-3596. doi: 10.1364/BOE.494620. eCollection 2023 Jul 1. Biomed Opt Express. 2023. PMID: 37497496 Free PMC article.
-
The role of NPY2R/NFATc1/DYRK1A regulatory axis in sebaceous glands for sebum synthesis.Cell Mol Biol Lett. 2023 Jul 27;28(1):60. doi: 10.1186/s11658-023-00467-4. Cell Mol Biol Lett. 2023. PMID: 37501148 Free PMC article.
-
Neuronal Reg3β/macrophage TNF-α-mediated positive feedback signaling contributes to pain chronicity in a rat model of CRPS-I.Sci Adv. 2025 Aug;11(31):eadu4270. doi: 10.1126/sciadv.adu4270. Epub 2025 Aug 1. Sci Adv. 2025. PMID: 40749060 Free PMC article.
-
Genome-Wide Expression Profiling by RNA-Sequencing in Spinal Cord Dorsal Horn of a Rat Chronic Postsurgical Pain Model to Explore Potential Mechanisms Involved in Chronic Pain.J Pain Res. 2022 Apr 5;15:985-1001. doi: 10.2147/JPR.S358942. eCollection 2022. J Pain Res. 2022. PMID: 35411184 Free PMC article.
-
Characterization of pain-related behaviors and gene expression profiling of peripheral sensory ganglia in a mouse model of acute ankle sprain.Front Behav Neurosci. 2023 May 25;17:1189489. doi: 10.3389/fnbeh.2023.1189489. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37304762 Free PMC article.
References
-
- Scheinman PL, Vocanson M, Thyssen JP, Johansen JD, Nixon RL, Dear K, Botto NC, Morot J, Goldminz AM. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. - PubMed
-
- Ohtaki N, Oka K, Sugimoto A, Akizawa T, Yasuhara T, Azuma H. Cutaneous reactions caused by experimental exposure to jellyfish, Carybdea rastonii. J Dermatol. 1990;17:108–114. - PubMed
-
- Fonacier LS, Dreskin SC, Leung DY. Allergic skin diseases. J Allergy Clin Immunol. 2010;125:S138–149. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

