A review on the diagnosis of animal trypanosomoses
- PMID: 35183235
- PMCID: PMC8858479
- DOI: 10.1186/s13071-022-05190-1
A review on the diagnosis of animal trypanosomoses
Abstract
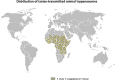
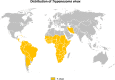
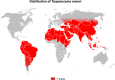
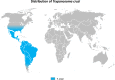
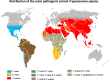
This review focuses on the most reliable and up-to-date methods for diagnosing trypanosomoses, a group of diseases of wild and domestic mammals, caused by trypanosomes, parasitic zooflagellate protozoans mainly transmitted by insects. In Africa, the Americas and Asia, these diseases, which in some cases affect humans, result in significant illness in animals and cause major economic losses in livestock. A number of pathogens are described in this review, including several Salivarian trypanosomes, such as Trypanosoma brucei sspp. (among which are the agents of sleeping sickness, the human African trypanosomiasis [HAT]), Trypanosoma congolense and Trypanosoma vivax (causing "Nagana" or animal African trypanosomosis [AAT]), Trypanosoma evansi ("Surra") and Trypanosoma equiperdum ("Dourine"), and Trypanosoma cruzi, a Stercorarian trypanosome, etiological agent of the American trypanosomiasis (Chagas disease). Diagnostic methods for detecting zoonotic trypanosomes causing Chagas disease and HAT in animals, as well as a diagnostic method for detecting animal trypanosomes in humans (the so-called "atypical human infections by animal trypanosomes" [a-HT]), including T. evansi and Trypanosoma lewisi (a rat parasite), are also reviewed. Our goal is to present an integrated view of the various diagnostic methods and techniques, including those for: (i) parasite detection; (ii) DNA detection; and (iii) antibody detection. The discussion covers various other factors that need to be considered, such as the sensitivity and specificity of the various diagnostic methods, critical cross-reactions that may be expected among Trypanosomatidae, additional complementary information, such as clinical observations and epizootiological context, scale of study and logistic and cost constraints. The suitability of examining multiple specimens and samples using several techniques is discussed, as well as risks to technicians, in the context of specific geographical regions and settings. This overview also addresses the challenge of diagnosing mixed infections with different Trypanosoma species and/or kinetoplastid parasites. Improving and strengthening procedures for diagnosing animal trypanosomoses throughout the world will result in a better control of infections and will significantly impact on "One Health," by advancing and preserving animal, human and environmental health.
Keywords: Antibody-detection; Cross-reactions; DNA detection; Microscope examination; Trypanosome; Undetected infection.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Murray M, Gray AR. The current situation on animal trypanosomiasis in Africa. Prev Vet Med. 1984;2:23–30. doi: 10.1016/0167-5877(84)90045-X. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

