International Association for the Study of Lung Cancer Study of the Impact of Coronavirus Disease 2019 on International Lung Cancer Clinical Trials
- PMID: 35183774
- PMCID: PMC8851565
- DOI: 10.1016/j.jtho.2022.01.017
International Association for the Study of Lung Cancer Study of the Impact of Coronavirus Disease 2019 on International Lung Cancer Clinical Trials
Abstract
Introduction: To evaluate the effects of the global coronavirus disease 2019 (COVID-19) pandemic on lung cancer trials, we surveyed investigators and collected aggregate enrollment data for lung cancer trials across the world before and during the pandemic.
Methods: A Data Collection Survey collected aggregate monthly enrollment numbers from 294 global lung cancer trials for 2019 to 2020. A 64-question Action Survey evaluated the impact of COVID-19 on clinical trials and identified mitigation strategies implemented.
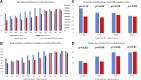
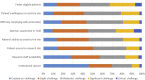
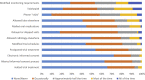
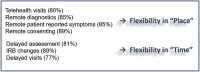
Results: Clinical trial enrollment declined from 2019 to 2020 by 14% globally. Most reductions in enrollment occurred in April to June where we found significant decreases in individual site enrollment (p = 0.0309). Enrollment was not significantly different in October 2019 to December of 2019 versus 2020 (p = 0.25). The most frequent challenges identified by the Action Survey (N = 172) were fewer eligible patients (63%), decrease in protocol compliance (56%), and suspension of trials (54%). Patient-specific challenges included access to trial site (49%), ability to travel (54%), and willingness to visit the site (59%). The most frequent mitigation strategies included modified monitoring requirements (47%), telehealth visits (45%), modified required visits (25%), mail-order medications (25%), and laboratory (27%) and radiology (21%) tests at nonstudy facilities. Sites that felt the most effective mitigation strategies were telehealth visits (85%), remote patient-reported symptom collection (85%), off-site procedures (85%), and remote consenting (89%).
Conclusions: The COVID-19 pandemic created many challenges for lung cancer clinical trials conduct and enrollment. Mitigation strategies were used and, although the pandemic worsened, trial enrollment improved. A more flexible approach may improve enrollment and access to clinical trials, even beyond the pandemic.
Keywords: COVID-19; Clinical trials; Lung cancer; Telehealth.
Copyright © 2022. Published by Elsevier Inc.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Early Impact of COVID-19 on the Conduct of Oncology Clinical Trials and Long-Term Opportunities for Transformation: Findings From an American Society of Clinical Oncology Survey.JCO Oncol Pract. 2020 Jul;16(7):417-421. doi: 10.1200/OP.20.00275. Epub 2020 May 12. JCO Oncol Pract. 2020. PMID: 32396491
-
COVID-19 and Clinical Trials: Current Challenges and Future Directions.Rev Recent Clin Trials. 2021;16(3):258-261. doi: 10.2174/1574887116666210122160705. Rev Recent Clin Trials. 2021. PMID: 33480349 Review.
-
Non-small cell lung cancer clinical trials requiring biopsies with biomarker-specific results for enrollment provide unique challenges.Cancer. 2017 Dec 15;123(24):4800-4807. doi: 10.1002/cncr.31056. Epub 2017 Nov 10. Cancer. 2017. PMID: 29125624 Free PMC article. Review.
Cited by
-
New Normal for Lung Cancer Clinical Trials Under Coronavirus Disease 2019.J Thorac Oncol. 2022 May;17(5):588-591. doi: 10.1016/j.jtho.2022.03.001. J Thorac Oncol. 2022. PMID: 35465962 Free PMC article. No abstract available.
-
Researcher Experience and Comfort With Telemedicine and Remote Patient Monitoring in Cancer Treatment Trials.Oncologist. 2024 Apr 4;29(4):356-363. doi: 10.1093/oncolo/oyad237. Oncologist. 2024. PMID: 37676048 Free PMC article.
-
Lung cancer in Asia: the impact of climate change.EClinicalMedicine. 2024 Jul 8;74:102680. doi: 10.1016/j.eclinm.2024.102680. eCollection 2024 Aug. EClinicalMedicine. 2024. PMID: 39764182 Free PMC article. Review.
-
Biological and Exploitable Crossroads for the Immune Response in Cancer and COVID-19.Biomedicines. 2022 Oct 19;10(10):2628. doi: 10.3390/biomedicines10102628. Biomedicines. 2022. PMID: 36289890 Free PMC article. Review.
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Zhang Y., Luo G., Etxeberria J., Hao Y. Global patterns and trends in lung cancer incidence: a population-based study. J Thorac Oncol. 2021;16:933–944. - PubMed

