The transcription factor complex CmAP3-CmPI-CmUIF1 modulates carotenoid metabolism by directly regulating carotenogenic gene CmCCD4a-2 in chrysanthemum
- PMID: 35184172
- PMCID: PMC9125392
- DOI: 10.1093/hr/uhac020
The transcription factor complex CmAP3-CmPI-CmUIF1 modulates carotenoid metabolism by directly regulating carotenogenic gene CmCCD4a-2 in chrysanthemum
Abstract
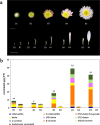
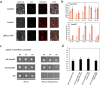
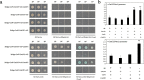
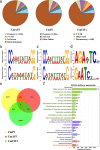
Carotenoids are one of the most important pigments for the coloring in many plants, fruits and flowers. Recently, significant progress has been made in carotenoid metabolism. However, the specific understanding on transcriptional regulation controlling the expression of carotenoid metabolic genes remains extremely limited. Anemone-type chrysanthemum, as a special group of chrysanthemum cultivars, contain elongated disc florets in capitulum, which usually appear in different colors compared with the ray florets since accumulating distinct content of carotenoids. In this study, the carotenoid composition and content of the ray and disc florets of an anemone-type chrysanthemum cultivar 'Dong Li Fen Gui' were analyzed by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) and the key structural gene CmCCD4a-2, of which differential expression resulted in the distinct content of carotenoids accumulated in these two types of florets, was identified. Then the promoter sequence of CmCCD4a-2 was used as bait to screen a chrysanthemum flower cDNA library and two transcription factors, CmAP3 and CmUIF1 were identified. Y2H, BiFC and Y3H experiments demonstrated that these two TFs were connected by CmPI to form CmAP3-CmPI-CmUIF1 TF complex. This TF complex regulated carotenoid metabolism through activating the expression of CmCCD4a-2 directly. Furthermore, a large number of target genes regulated directly by the CmAP3-CmPI-CmUIF1 TF complex, including carotenoid biosynthetic genes, flavonoid biosynthetic genes and flower development-related genes, were identified by DNA-affinity purification sequencing (DAP-seq), which indicated that the CmAP3-CmPI-CmUIF1 TF complex might participate in multiple processes. These findings expand our knowledge for the transcriptional regulation of carotenoid metabolism in plants and will be helpful to manipulating carotenoid accumulation in chrysanthemum.
Keywords: CmCCD4a-2; CmUIF1-CmPI-CmAP3 transcription factor complex; carotenoid; chrysanthemum; transcriptional regulation.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Comparative transcriptomics and weighted gene co-expression correlation network analysis (WGCNA) reveal potential regulation mechanism of carotenoid accumulation in Chrysanthemum × morifolium.Plant Physiol Biochem. 2019 Sep;142:415-428. doi: 10.1016/j.plaphy.2019.07.023. Epub 2019 Aug 2. Plant Physiol Biochem. 2019. PMID: 31416008
-
The promoter of the carotenoid cleavage dioxygenase 4a-5 gene of Chrysanthemum morifolium (CmCCD4a-5) drives petal-specific transcription of a conjugated gene in the developing flower.J Plant Physiol. 2013 Sep 15;170(14):1295-9. doi: 10.1016/j.jplph.2013.04.001. Epub 2013 May 2. J Plant Physiol. 2013. PMID: 23643306
-
Flower color mutation, pink to orange, through CmGATA4 - CCD4a-5 module regulates carotenoids degradation in chrysanthemum.Plant Sci. 2022 Sep;322:111290. doi: 10.1016/j.plantsci.2022.111290. Epub 2022 Apr 20. Plant Sci. 2022. PMID: 35753140
-
Molecular mechanisms underlying the diverse array of petal colors in chrysanthemum flowers.Breed Sci. 2018 Jan;68(1):119-127. doi: 10.1270/jsbbs.17075. Epub 2018 Feb 17. Breed Sci. 2018. PMID: 29681754 Free PMC article. Review.
-
Regulation of Carotenoid Biosynthesis During Fruit Development.Subcell Biochem. 2016;79:161-98. doi: 10.1007/978-3-319-39126-7_6. Subcell Biochem. 2016. PMID: 27485222 Review.
Cited by
-
Genome-wide identification of the MIKCc-type MADS-box gene family in Chrysanthemum lavandulifolium reveals their roles in the capitulum development.Front Plant Sci. 2023 Mar 23;14:1153490. doi: 10.3389/fpls.2023.1153490. eCollection 2023. Front Plant Sci. 2023. PMID: 37035079 Free PMC article.
-
Integrative Analysis of Metabolome and Transcriptome Revealed Lutein Metabolism Contributed to Yellow Flower Formation in Prunus mume.Plants (Basel). 2023 Sep 21;12(18):3333. doi: 10.3390/plants12183333. Plants (Basel). 2023. PMID: 37765497 Free PMC article.
-
Expression variation of Viola APETALA3 orthologous genes is correlated with chasmogamous and cleistogamous flower development.BMC Plant Biol. 2025 Mar 12;25(1):319. doi: 10.1186/s12870-025-06348-6. BMC Plant Biol. 2025. PMID: 40075258 Free PMC article.
-
cla-miR164-NO APICAL MERISTEM (ClNAM) regulates the inflorescence architecture development of Chrysanthemum lavandulifolium.Hortic Res. 2024 Feb 22;11(4):uhae039. doi: 10.1093/hr/uhae039. eCollection 2024 Apr. Hortic Res. 2024. PMID: 38623074 Free PMC article.
-
A 49-bp deletion of PmAP2L results in a double flower phenotype in Prunus mume.Hortic Res. 2023 Dec 19;11(2):uhad278. doi: 10.1093/hr/uhad278. eCollection 2024 Feb. Hortic Res. 2023. PMID: 38371636 Free PMC article.
References
-
- Rodriguez-Concepcion M, Avalos J, Bonet MLet al. . A global perspective on carotenoids: metabolism, biotechnology, and benefits for nutrition and health. Prog Lipid Res. 2018;70:62–93. - PubMed
-
- Hashimoto H, Uragami C, Cogdell RJ. Carotenoids and photosynthesis. Subcell Biochem. 2016;79:111–39. - PubMed
-
- Alder A, Jamil M, Marzorati Met al. . The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335:1348–51. - PubMed
-
- Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. 2018;652:18–26. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

