Tea plant-legume intercropping simultaneously improves soil fertility and tea quality by changing bacillus species composition
- PMID: 35184199
- PMCID: PMC9123240
- DOI: 10.1093/hr/uhac046
Tea plant-legume intercropping simultaneously improves soil fertility and tea quality by changing bacillus species composition
Abstract
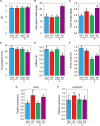
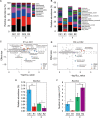
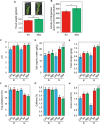
Tea plant is an economically important crop in China, but long-term monoculture and substantial chemical nitrogen fertilizer input cause soil acidification, which in turn affects the nutrient supply and tea quality. Intercropping has drawn more attention in tea gardens because this pattern is expected to improve soil fertility and tea quality and change the soil microbial community composition. However, the roles of some key microorganisms in rhizosphere soils have not been well characterized. Hereby, a "soybean in summer and smooth vetch in winter" mode was selected to investigate the effects of intercropped legumes in a tea garden on soil fertility, tea quality, and the potential changes in beneficial bacteria such as Bacillus. Our data showed that when soybeans were turned into soil, intercropping system exhibited higher soil organic matter (SOM), total nitrogen (TN), tea quality indices and the expression of Camellia sinensis glutamine synthetase gene (CsGS). Notably, intercropping significantly affected the bacterial communities and decreased the relative abundance of Bacillus but increased its absolute abundance. Bacillus amyloliquefaciens BM1 was isolated from intercropped soil and showed outstanding plant growth-promoting (PGP) properties when coinoculated with rhizobia. In winter, intercropping with smooth vetch had a beneficial effect on soil properties and tea quality. Comparably, coinoculation with strain BM1 and Rhizobium leguminosarum Vic5 on smooth vetch (Vicia villosa) showed huge improvements in SOM, TN and quality of tea leaves, accompanied by the highest level of amino acids and lowest levels of polyphenol and caffeine (p < 0.05). According to these results, our findings demonstrate that intercropping with some legumes in the tea garden is a strategy that increases SOM, TN and tea quality, and some PGP Bacillus species are optional to obtain an amplification effect.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved.
Figures


References
-
- Silva LS, Seabra AR, Leitão JN, Carvalho HG. Possible role of glutamine synthetase of the prokaryotic type (GSI-like) in nitrogen signaling in Medicago truncatula. Plant Sci. 2015;240:98–108. - PubMed
-
- Rana NK, Mohanpuria P, Yadav SK. Cloning and characterization of a cytosolic glutamine synthetase from Camellia sinensis (L.) O. Kuntze that is upregulated by ABA, SA, and H2O2. Mol Biotechnol. 2008;39:49–56. - PubMed
-
- Wu Y, Li Y, Fu Xet al. . Three-dimensional spatial variability in soil microorganisms of nitrification and denitrification at row-transect scale in a tea field. Soil Biol Biochem. 2016;103:452–63.
-
- Ma L, Chen H-j, Shan Y-jet al. . Status and suggestions of tea garden fertilization on main green tea-producing counties in Zhengjiang Province. J Tea Sci. 2013;33:74–84.
-
- Li Y, Li ZW, Arafat Yet al. . Characterizing rhizosphere microbial communities in long-term monoculture tea orchards by fatty acid profiles and substrate utilization. Eur J Soil Biol. 2017;81:48–54.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

