Regulatory mechanisms of the early phase of white adipocyte differentiation: an overview
- PMID: 35184223
- PMCID: PMC8858922
- DOI: 10.1007/s00018-022-04169-6
Regulatory mechanisms of the early phase of white adipocyte differentiation: an overview
Abstract
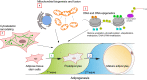
The adipose organ comprises two main fat depots termed white and brown adipose tissues. Adipogenesis is a process leading to newly differentiated adipocytes starting from precursor cells, which requires the contribution of many cellular activities at the genome, transcriptome, proteome, and metabolome levels. The adipogenic program is accomplished through two sequential phases; the first includes events favoring the commitment of adipose tissue stem cells/precursors to preadipocytes, while the second involves mechanisms that allow the achievement of full adipocyte differentiation. While there is a very large literature about the mechanisms involved in terminal adipogenesis, little is known about the first stage of this process. Growing interest in this field is due to the recent identification of adipose tissue precursors, which include a heterogenous cell population within different types of adipose tissue as well as within the same fat depot. In addition, the alteration of the heterogeneity of adipose tissue stem cells and of the mechanisms involved in their commitment have been linked to adipose tissue development defects and hence to the onset/progression of metabolic diseases, such as obesity. For this reason, the characterization of early adipogenic events is crucial to understand the etiology and the evolution of adipogenesis-related pathologies, and to explore the adipose tissue precursors' potential as future tools for precision medicine.
Keywords: Adipogenesis; Cytoskeleton; Epigenome modifications; Obesity; Transcriptional control of differentiation.
© 2022. The Author(s).
Conflict of interest statement
Authors declare no conflict of interest.
Figures
Similar articles
-
The expanding problem of adipose depot remodeling and postnatal adipocyte progenitor recruitment.Mol Cell Endocrinol. 2017 Apr 15;445:95-108. doi: 10.1016/j.mce.2016.10.011. Epub 2016 Oct 12. Mol Cell Endocrinol. 2017. PMID: 27743993 Free PMC article. Review.
-
White adipose remodeling during browning in mice involves YBX1 to drive thermogenic commitment.Mol Metab. 2021 Feb;44:101137. doi: 10.1016/j.molmet.2020.101137. Epub 2020 Dec 5. Mol Metab. 2021. PMID: 33285300 Free PMC article.
-
Irisin exerts dual effects on browning and adipogenesis of human white adipocytes.Am J Physiol Endocrinol Metab. 2016 Aug 1;311(2):E530-41. doi: 10.1152/ajpendo.00094.2016. Epub 2016 Jul 19. Am J Physiol Endocrinol Metab. 2016. PMID: 27436609
-
Fifty shades of white: Understanding heterogeneity in white adipose stem cells.Adipocyte. 2017 Jul 3;6(3):205-216. doi: 10.1080/21623945.2017.1372871. Epub 2017 Sep 12. Adipocyte. 2017. PMID: 28949833 Free PMC article. Review.
-
Cell-Autonomous Brown-Like Adipogenesis of Preadipocytes From Retinoblastoma Haploinsufficient Mice.J Cell Physiol. 2016 Sep;231(9):1941-52. doi: 10.1002/jcp.25299. Epub 2016 Jan 14. J Cell Physiol. 2016. PMID: 26727985
Cited by
-
White Adipocyte Stem Cell Expansion Through Infant Formula Feeding: New Insights into Epigenetic Programming Explaining the Early Protein Hypothesis of Obesity.Int J Mol Sci. 2025 May 8;26(10):4493. doi: 10.3390/ijms26104493. Int J Mol Sci. 2025. PMID: 40429638 Free PMC article. Review.
-
Human Differentiated Adipocytes as Surrogate Mature Adipocytes for Adipocyte-Derived Extracellular Vesicle Analysis.Cells. 2025 May 22;14(11):757. doi: 10.3390/cells14110757. Cells. 2025. PMID: 40497934 Free PMC article.
-
Concentration of Polycyclic Aromatic Hydrocarbons (PAHs) in Human Serum and Adipose Tissues and Stimulatory Effect of Naphthalene in Adipogenesis in 3T3-L1 Cells.Int J Mol Sci. 2023 Jan 11;24(2):1455. doi: 10.3390/ijms24021455. Int J Mol Sci. 2023. PMID: 36674971 Free PMC article.
-
Ectopic lipid metabolism in anterior pituitary dysfunction.Front Endocrinol (Lausanne). 2023 Feb 13;14:1075776. doi: 10.3389/fendo.2023.1075776. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36860364 Free PMC article. Review.
-
Management of Obesity and Obesity-Related Disorders: From Stem Cells and Epigenetics to Its Treatment.Int J Mol Sci. 2023 Jan 24;24(3):2310. doi: 10.3390/ijms24032310. Int J Mol Sci. 2023. PMID: 36768633 Free PMC article. Review.

