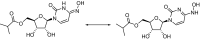Molnupiravir: First Approval
- PMID: 35184266
- PMCID: PMC8858220
- DOI: 10.1007/s40265-022-01684-5
Molnupiravir: First Approval
Abstract
Molnupiravir (Lagevrio®) is an orally-administered antiviral prodrug that inhibits replication of RNA viruses through viral error induction. It is being developed by Merck and Ridgeback Biotherapeutics for the prevention and treatment of Coronavirus disease 2019 (COVID-19). Molnupiravir received its first approval on 4 November 2021 in the UK for the treatment of mild to moderate COVID-19 in adults with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnostic test and who have at least one risk factor for developing severe illness. Molnupiravir is filed for approval and has emergency use authorization for the treatment of COVID-19 in several countries, including the USA, Japan and those in the EU. This article summarizes the milestones in the development of molnupiravir leading to this first approval for COVID-19.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous